Testing for Biological Molecules
-
- Positive: green → yellow → orange → brick red
- Negative: blue
Acid or enzyme hydrolysis followed by Benedict’s test for non-reducing sugars
Hydrochloric acid (catalyst) is added to hydrolyse the sugar in the sample being tested in the ratio of 1:2 respectively and heated in a water bath for approximately 2 minutes. A pinch of sodium hydroxide is added to make the solution alkaline. After this, Benedict’s test is carried out.
Biuret’s test used to detect the presence of proteins
Equal amounts of the sample and Biuret’s solution are added together, giving purple colour over several minutes in the presence of proteins, and blue in its absence.
Emulsion test for lipids
The sample is added to 2cm3 of ethanol and mixed well until it dissolves (lipids are soluble in ethanol). This mixture is then placed into a test tube containing the same amount of water. A milky white emulsion will appear if lipids are present and remain clear if not.
Iodine test for the presence of starch
Iodine solution is orange-brown. Add a drop of iodine solution to the solid or liquid substance to be tested. A blue-black colour is quickly produced if starch is present.
Carbohydrates and Lipids
Glucose has the molecular formula C6H12O6. It is an energy source which is broken down during respiration. It is also the monomer from which Starch and Cellulose are made. There are two different kinds of glucose monomers known as α- glucose and β – glucose and their difference lies between the position of an –OH group in their ring structures.


Monomer
simple molecule which is used as a basic building block for the synthesis of a polymer; many monomers are joined together to make the polymer, usually by condensation reactions e.g. monosaccharides, amino acids, nucleotide.
Polymer
is a giant molecule made from monomers e.g. polysaccharides, proteins, nucleic acids
Macromolecule
These are large and complex molecules that are formed due to polymerisation of smaller monomers e.g. polysaccharides, nucleic acids
Monosaccharide
This is a molecule consisting a single sugar unit, the simplest form of carbohydrate and cannot be hydrolysed further. It has a general formula of (CH2O)n.
Disaccharide
is a sugar molecule consisting of two monosaccharides joined together by a glycosidic bond.
Polysaccharide
a polymer whose subunits are monosaccharides joined together by glycosidic bonds.
Glycosidic bonds
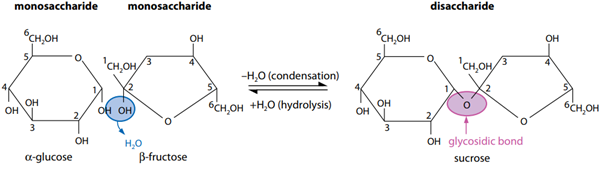
Starch
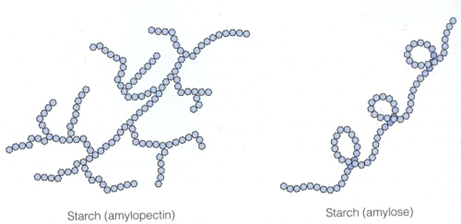
is a macromolecule that is found in plant cells and is made up of two components known as amylose and amylopectin. These components are polysaccharides that are made from a glucose molecules and contain 1,4 glycosidic bonds. Starch is highly compact and stores energy.
| Amylose | Amylopectin | |
| Structure | α 1,4 glycosidic bonds | α 1,4 and α 1,6 glycosidic bonds, giving it’s branched structure |
| Shape | Helical and more compact | Branched |
Glycogen
a macromolecule that is used for the storage of energy is animal cells and is also made from α glucose molecules. The structure of glycogen is very similar to that of amylopectin; however, it is more branched and therefore contains more α 1,6 glycosidic bonds.
Cellulose
found in the cell wall of plant cells and is made from βglucose units that form β-1,4 glycosidic bonds. Alternate β- glucose molecules are rotated 180 degrees in order to form these bonds.
- Hydrogen bonds are also formed between parallel cellulose molecules. 60 and 70 cellulose molecules become tightly cross-linked to form bundles called microfibrils. Microfibrils are in turn held together in bundles called fibres by hydrogen bonding.
- Fibres increase tensile strength to withstand osmotic pressure, making the plant rigid and determine cell shape. They’re also freely permeable…

Triglyceride
-
- Unsaturated fatty acids: contain c=c bonds that are easier to break and melt easily. More than one c=c is a polyunsaturated fatty acid.
- Saturated fatty acids: contain c-c bonds that are solids at room temperature.
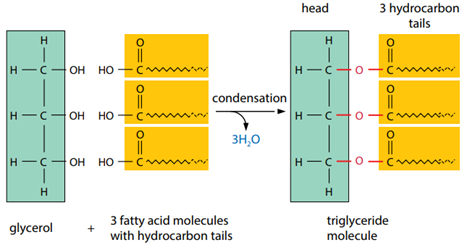
Role of triglyceride
- Better energy reserves than carbohydrates as more CH bonds
- Acts as an insulator and provides buoyancy
- A metabolic source of water as gives CO2 and H20 on oxidation in respiration
Phospholipid
The hydrophilic head contains a phosphate group and glycerol while the hydrophobic tail contains 2 fatty acid chains. This is due to the partial negative charge on the phosphate group that gets attracted to the partial positive charge on the hydrogen atom of the water molecule.

- Role of phospholipids: (ref Cell Membrane and Transport > Fluid Mosaic Membranes > phospholipids)
Proteins
Proteins are made of amino acids which only differ in the R- groups/ variable side chains and will always contain an amine group (basic), carboxyl group (acidic) and a hydrogen atom attached to the central carbon atom.

- A peptide bond is formed by condensation between 2 amino acids, forming a dipeptide. Many amino acids that join together by peptide bonds form a polypeptide.
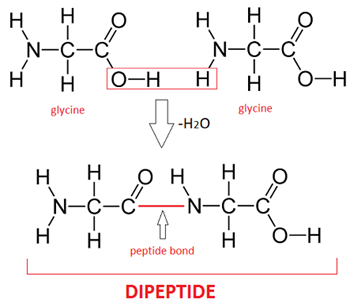
- Peptide bonds are broken when hydrolysed into amino acids, often occurring in the small intestine and stomach.
Protein Structure
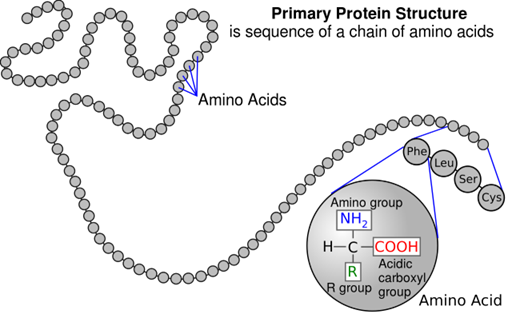
Primary structure
sequence of amino acids in a polypeptide/protein. A slight change in the sequence of amino acids can affect the protein’s structure and function. It has a unique sequence for each protein.
Secondary structure:
-
- α- helix: the polypeptide chain twists into a regular spiral and is maintained by hydrogen bonds between the (-NH) group of one amino acid and the (CO-) group of another amino acid 4 spaces later in the polypeptide chain.
- β- pleated sheet: the chain is not tightly coiled and lies in a looser, straighter shape.

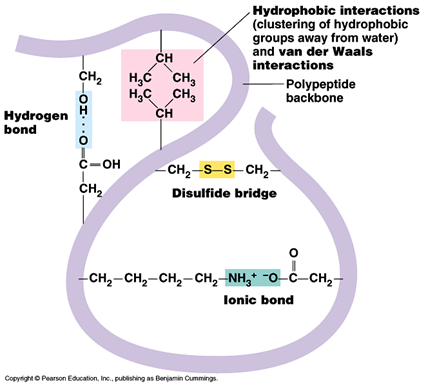
Tertiary structure
-
- Hydrogen bonds between wide varieties of R- groups (can be broken by PH and temperature changes)
- Disulphide bridges between two cysteine molecules (can be broken by reducing agents)
- Ionic bonds between R groups containing amine and carboxyl groups. (Can be broken by PH changes.)
- Hydrophobic interactions between non polar R groups.
Quaternary structure:
the three-dimensional arrangement of two or more polypeptides, or of a polypeptide and a non-protein component such as haem, in a protein molecule. The polypeptide chains are held together by bonds in the tertiary structure.
Globular proteins
curl up into a spherical shape with their hydrophobic regions pointing into the centre of the molecule and hydrophilic regions pointing outwards. They are soluble in water e.g. enzymes and hemoglobin.
Fibrous proteins
form long strands, are insoluble in water, and have structural roles e.g. collagen, hair, nails.
Haemoglobin
a globular protein that has a quaternary structure with 4 polypeptide chains, 2 α-globin and 2 β-globin chains. Each chain has one prosthetic haem group containing an iron atom that reversibly binds to an oxygen molecule. Oxyhaemoglobin is bright red, when the haem group is combined with oxygen, otherwise it’s purplish.

Sickle cell anaemia:
- is a genetic condition in which a polar amino acid, glutamic acid is substituted by non-polar valine on the surface of the β chain in hemoglobin, making it less soluble.
Collagen
-
- A collagen molecule has 3 polypeptide chains that are coiled in the shape of a stretched-out helix.
- Compact structure and almost every 3rd amino acid is glycine, the smallest amino acid which can form H-bonds.
- 3 polypeptide strands are held together by hydrogen and covalent bonds.
- Many of these collagen molecules lie side by side, linked to each other by covalent cross-links between the side chains of amino acids, forming fibrils, and many fibrils make up a fibre.


Water
Hydrogen bonding
A water molecule contains two hydrogen atoms and one oxygen atom held together by hydrogen bonds.
Solvent
Water is an effective solvent because of its polarity and so can form electrostatic interactions with other polar molecules and ions. Thus it’s a transport medium and reagent for metabolic and other reactions in the cells of plants and animals.
High surface tension and cohesion
cohesion refers to the attraction of one water molecule to the other. Water molecules have strong cohesive forces due to hydrogen bonds, thus having high surface tension.
High specific heat capacity
the amount of heat energy required to raise the temperature of 1 kg of water by 1 °C. Water has high SPC due to its hydrogen bonds. Temperature within organisms remains constant compared to external temperature, and water bodies also have a slow change in temperature, providing stable aquatic habitats.
High latent heat of vaporization
measure of the heat energy needed to vaporise a liquid. Water has a high LHV due to its high SPC as H bonds need to be broken before water can be vaporised, cooling the surrounding environment. Sweating is a good cooling mechanism. However, a large amount of energy can be lost for little amount of water, thus dehydration is prevented e.g. in transpiration.
Density and freezing properties
ice is less dense than water and floats on it, insulating water and preventing it from freezing, preserving aquatic life underneath it. Changes in the density of water with temperature cause currents, which help to maintain the circulation of nutrients in the oceans.