Acid halides are highly reactive carboxylic acid derivatives. As such they are able to be used to synthesize many other carboxylic acid derivatives.
Formation of Acid Halides
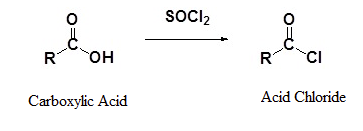
Carboxylic acids react with thionyl chloride (SOCl2) to form acid chlorides. A nucleophilic acyl substitution allows for the replacement of the carboxylic acid –OH with a chloride atom.
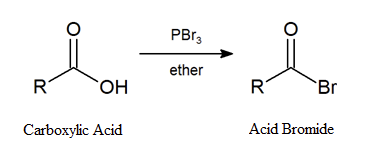
Primary and secondary alcohols react with phosphorous tribromide (PBr3) forming the corresponding alkyl bromide. Similarly, acid bromides can be formed from the corresponding carboxylic acid by reaction with PBr3.
Reactions of Acid Halides
The high reactivity of acid halides allows them to be easily converted into other acyl compound through nucleophilic acyl substitution. Depending on the nucleophilic reagent applied, acid halides can be used to form:
- carboxylic acids
- anhydrides
- esters
- amides
- ketones.
Also, acid halides undergo a double nucleophilic addition with LiAlH4 to produce primary alcohols and Grignard reagents to produce tertiary alcohols.
Conversion of Acid Chlorides to Carboxylic Acids
Hydrolysis
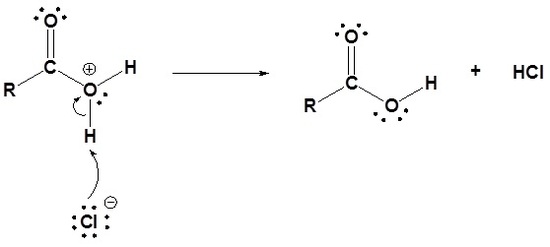
- Acid chlorides are converted into carboxylic acids through a nucleophilic acyl substitution with water.
- The carbonyl bond is reformed and Cl- is eliminated as a leaving group.
- as water is a neutral nucleophile, an oxonium intermediate in produced.
- The oxonium intermediate is deprotonated by the chloride anion to produce a neutral carboxylic acid and HCl.
General reaction
Example
Mechanism
1) Nucleophilic attack by water
2) Leaving group is removed
3) Deprotonation
Conversion of Acid Chlorides to Anhydrides
- Acid chlorides react with carboxylic acids to form anhydrides through a nucleophilic acyl substitution.
- as the carboxylic acid nucleophile is neutral, HCl is produced as a side-product during the reaction and is typically removed as part of a basic work-up.
- Both symmetrical and asymmetrical anhydrides can be created using this reaction.
General Reaction
Example
Mechanism
1) Nucleophilic attack by a carboxylic acid
2) Leaving group removal
3) Deprotonation
Conversion of Acid Chlorides to Esters
Acid chlorides react with alcohol nucleophiles to produce esters. This reaction is the preferred method for preparing esters.
General Reaction
Example
Mechanism
1) Nucleophilic attack by an alcohol
2) Leaving group removal
3) Deprotonation
Conversion of Acid Chlorides to Aldehydes
Reduction
- Acid chlorides can be converted to aldehydes using a hindered reducing agent such as lithium tri-tert-butoxyaluminum hydride LiAlH(Ot-Bu)3 or diisobutylaluminum hydride (DIBALH).
- other hydride sources are weaker reducing agents than lithium aluminum hydride in part because they are sterically hindered.
General Reaction
Example
Conversion of Acid chlorides to Amides
- Ammonia, 1o amines, and 2o amines react with acid chlorides to form 1o, 2o, and 3o amides respectively.
- These reactions typically take place rapidly at room temperature and provides high reaction yields.
General Reaction
Examples
Mechanism
- it follows a typical nucleophilic acyl substitution.
- The amine nucleophile attacks the carbonyl carbon of the acid chloride forming an alkoxide tetrahedral intermediate.
- The -Cl leaving group is eliminated, allowing the carbonyl bond to be reformed. Because amines are neutral nucleophiles a protonated amide is produced after this step.
- Lastly, a second amine acts as a base, removing a proton, and allowing for the amide product to be formed.
1) Nucleophilic attack by an amine
2) Leaving group removal
3) Deprotonation