These are compounds in which an amino (NH2) group and a carboxyl group are attached to the same carbon atom (alpha-amino acids):
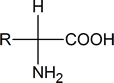
Such compounds contain a chiral carbon atom (unless R = H, which is glycine). Proteins can be considered to consist of amino acid residues joined by amide (or peptide) links. These peptide links consist of exactly the same structural units that we find in N‑substituted primary amides.
Preparation of Amides
- Amides are most commonly prepared though the reaction of an acid chloride with ammonia, a 1o amine, or 2o amine.
- an amide can be prepared through the reaction of a carboxylic acid and an amine using a coupling agent such as DCC.
- Simple amides can be prepared by reacting an acid anhydride with an amine.
- Amides can be formed through the direct reaction of a carboxylic acid and an amine.
Reactions of Amides
Amides are relatively unreactive towards nucleophilic acyl substitutions due to the poor leaving group ability of its nitrogen containing Y group. Despite this, amides can:
- react with water under acidic or basic conditions to create a carboxylic acid through nucleophilic acyl substitution.
- Reaction of primary amides with thionyl chloride (SOCl2) creates nitriles.
- Hydride reduction using LiAlH4 causes the carbonyl oxygen of the amide to eliminate as a leaving group creating the corresponding amine.
Conversion of Amides to Carboxylic Acids: Hydrolysis
Amides can be hydrolyzed into a carboxylic acid and ammonia or an amine by heating in an acidic or basic aqueous solution. In both cases, acid-base reactions occurring with the products make the overall reaction irreversible.
- Under acidic conditions the amine produced by the reaction is protonated to form a non-nucleophilic ammonium compound.
- Under basic conditions the carboxylic acid produced by the reaction is deprotonated to a non-electrophilic carboxylate.
General Reaction
Examples
Mechanism Under Acidic Conditions
1) Protonation of the carbonyl
Protonation of the amide increases the electrophilicity of the carbonyl carbon allowing water to add causing formation of a tetrahedral oxonium intermediate.
2) Nucleophilic attack by water
3) Proton transfer
a proton is transferred to the amide nitrogen giving it a positive charge and increasing its ability to act as a leaving group. Reforming the carbonyl double bond causes the elimination of an amine as a leaving group, creating a protonated carboxylic acid.
4) Leaving group removal
5) Deprotonation
The produced amine acts as a base, removing a hydrogen, to form a carboxylic acid and an ammonium compound.
Mechanism Under Basic Conditions
1) Nucleophilic attack by hydroxide
Nucleophilic addition of a hydroxide ion at the carbonyl carbon creating a tetrahedral alkoxide intermediate.
2) Leaving group removal
The carbonyl bond is reformed along with the elimination of an amide ion (-NHR) leaving group (note this is a different use of the word amide meaning an amine anion), forming a carboxylic acid.
3) Deprotonation
The amide ion deprotonates the carboxylic acid to form a carboxylate salt and an amine as products.
Conversion of 1o Amides to Nitriles
Dehydration
1o Amides can be converted into a nitrile through reaction with thionyl chloride (SOCl2). Sulfurdioxide (SO2) and hydrochloric acid are produced as byproducts.
General Reaction
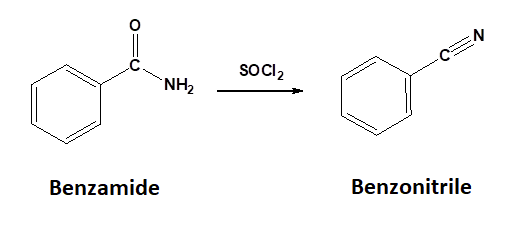
Mechanism for the Conversion of a 1o Amide to a Nitrile
1) Nucleophilic attach on thionyl chloride
The amide acts as a nucleophile and attacks the electrophilic sulfur of thionyl chloride, pushing the pi electrons of the S=O bond onto oxygen.
2) Leaving group removal
Removal of a chloride anion as a leaving group allows the sulfoxide S=O bond to reform creating a protonated imine chlorosulfite.
3) Deprotonation
Deprotonation forms an imine chlorosulfite intermediate.
4) Leaving group removal
A second deprotonation eliminates the chlorosulfite leaving group as sulfur dioxide and a chloride anion and forms the C-N triple bond of the nitrile product.
Conversion of Amides to 1o, 2o or 3o Amines:
Amides are reduced to amines by treatment with LiAlH4, and this one of the most general methods for preparing of amines (1º, 2º & 3º).
General Reaction
Predicting the Products of a Hydride Reduction
During this reaction, there are two major changes in bonding:
1) The C=O carbonyl is removed from the starting material.
2) Two hydride nucleophiles are added to what was the carbonyl carbon of the amide.
Example 1.
Alkyl groups attached to the nitrogen do not affect the reaction.