The covalent bond
A covalent bond is a chemical bonding formed by sharing of pairs of electrons between two atoms.
Lewis Theory (Octet rule)
Rule: A stable arrangement is attended when the atom is surrounded by eight electrons. This octet can be made up by own electrons and some electrons which are shared. Thus, an atom continues to form bonds until an octet of electrons is made. This is known as octet rule by Lewis.
Example:
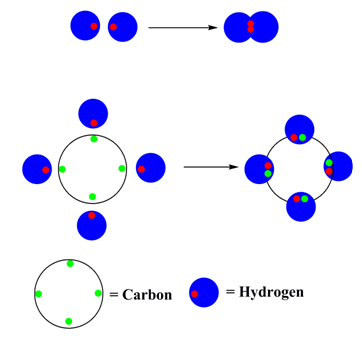
(i) Normally two electrons pairs up and forms a bond. Ex: H2
(ii) For most atoms there will be a maximum of eight electrons in the valence shell (octet structure). Ex: CH4
Exception of the octet rule
(A) PF 3 maintains octet rule, while PF 5 does not. PF 5 has ten outer electrons.
(B) Molecules comprised of odd number of electrons do not follow octet rule. Ex: NO, ClO2 , etc. This rule even does not explain the origin of paramagnetic character in O2 molecule.
(C) For atoms like Be and B which have less than four outer electrons. Even if all the outer electrons used to form bonds an octet will not be resulted for Be and B.
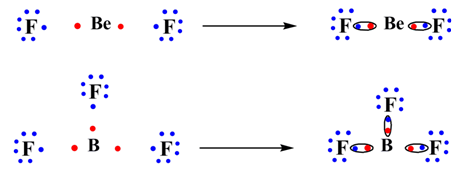
The octet rule has been further extended to include the exceptional part. Hence, the following rules are added to the octet rules.
(iii) For elements with available d orbitals, the valence shell can be expanded beyond an octet.
This rule explained the formation of PF 5 . Here P has low-energy d orbitals.
(iv) The molecule will seek the lowest overall energy. This means the maximum number of bonds will form, that the strongest possible bonds will form, and that the arrangement of the atoms in molecule will be in such as to minimize adverse repulsion energy.
Valence Bond Theory
Atoms containing unpaired electron(s) tend to combine with other atom(s) which also possess(es) unpaired electron(s). In this process unpaired electron(s) paired up and attained a stable noble gas configuration. Two electrons shared by two atoms constitute a bond. The number of bonds formed by an atom is same as the number of unpaired electrons in the ground state (lowest energy state). However, in some cases the atom may form more bonds than that of its acquired unpaired electron. This takes place by the excitation of an atom that promotes paired-electrons in the ground state to the excited state in a suitable empty orbital as unpaired electron.
The shape of a molecule is preliminary determined by the direction in which the participating orbitals point. Electrons in the valence shell of the original orbital are known as lone pairs.
A covalent bond is resulted by pairing of electrons from each atom. In this case the spin of the electrons must be opposite to each other.
Limitations
(i) Relative stability of different molecules cannot be explained.
(ii) Paramagnetic nature of complexes or molecules cannot be explained.
(iii) Different shape of molecules cannot be explained.
Hybridization
Hybridization is the mixing of atomic orbitals prior to overlap that change the partial distribution of orbitals.
Due to this hybridization mainly two different kind of bonds forms, (i) sigma (σ ) and (ii) pi (π) bond.
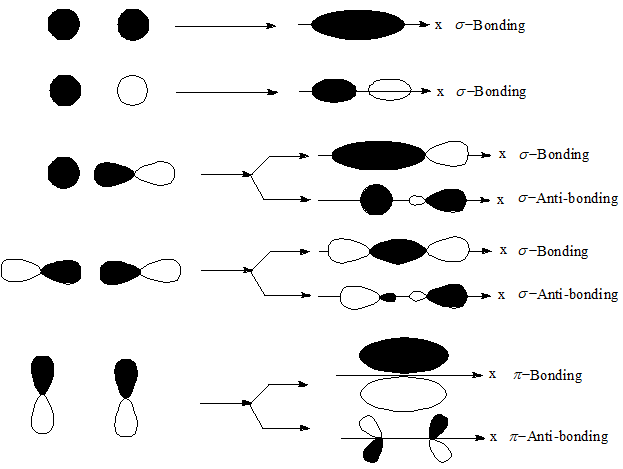
Sigma (σ) and pi (π) bonding and anti – bonding by the combination of s and p orbitals.
Sigma (σ) bond: A covalent bond established between two atoms having the maximum density of the electron cloud along the axis connecting the centers of the two participating atoms is called sigma (σ) bond.
Pi (π) bond: A bond is formed by the lateral overlap between two atomic orbitals possessing maximum electron density on both sides of the overlapping axis is known as pi (π) bond.
Therefore, from the definition it is clear that sigma (σ) bonds are more strong than pi (π) bonds.
Sigma (σ) bonds may arises from the overlap between s, p, and d orbitals, like (i) s – s orbitals, (ii) s – p prbitals, (iii) p – p orbitals, (iv) s – d orbitals, so on.
s orbitals are spherical, while p , d orbitals are dumbbell shaped. Hence, s – s over lap is weaker compared to s – p , p – p overlaps.
**Herein, it is important to note that due to hybridization of two atomic orbitals two molecular orbitals formed. One is called bonding orbital and the other is called anti – bonding orbital. Hence, n number of atomic orbitals forms n /2 bonding and n /2 anti – bonding orbitals.
Examples:
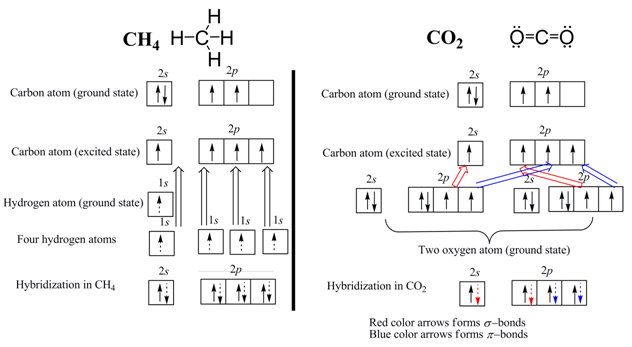
In the formation of CH4 one s orbital and three p orbital of the central carbon atom participate.
Similarly, in the CO2 molecule one s orbital and three p orbital of the central carbon atom participate but here two p orbitals of carbon atom forms two π – bonds. Therefore, CH4 is sp 3 hybridized and CO2 is sp 2 hybridized. NOTE: Orbitals participate in π – bond formation is not counted to the hybridization.