Redox principles involved in extraction of elements:
Electrochemical cells:
A device producing an electric current from a chemical reaction (Redox reaction) is called electrochemical cell i.e. it converts chemical energy to electrical energy.
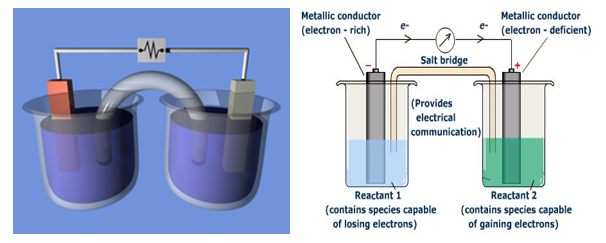
A demonstration electrochemical cell setup resembling the Daniell cell. The two half-cells are linked by a salt bridge carrying ions between them. Electrons flow in the external circuit.
A redox reaction consists of two half reactions called oxidation half reaction and reduction half reaction.
![]()
Two half redox reactions are

Electrochemical cell based on this reaction is called Daniel cell.
In electrochemical cell those two half reactions are used as two half cells joined together by salt bridge.
Salt bridge: A U-shaped tube containing a concentrated solution of an inert electrolyte like K2SO4 , KCl, KNO3 etc. The function of salt bridge is to complete inert circuit by flow of ions and to maintain the electrical neutrality in the solution of two half cells.
For making the salt bridge only those electrolytes are used for which cations and anions have nearly same ionic mobility.
In electrochemical cell, the electrode at which oxidation takes place is called anode or negative pole and the electrode at which reduction takes place is called anode or positive pole.
Electrochemical cell is represented as electron flow from anode to cathode in the external circuit while current flows from cathode to anode.
Electrode potential or half-cell potential:
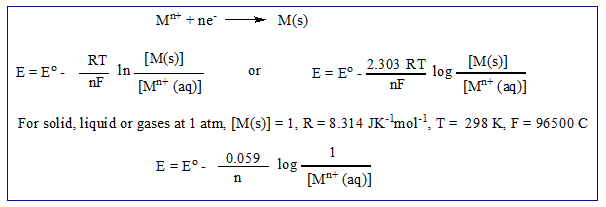
The tendency of an electrode to lose electrons is called oxidation potential, while tendency of an electrode to gain electrons is called reduction potential. Electrode potential depends upon concentration of metal ion and temperature. At standard condition i.e. 1 molar concentration and 298k are called standard electrode potential.
The absolute value of electrode potential cannot be measured directly because half reaction cannot take place independently. Electrode potential is measured against reference electrode i.e. standard hydrogen electrode (S.H.E) and the standard electrode potential of S.H.E is taken as zero. S.H.E is represented as
![]()
Standard electrode potential (E°) is given a positive sign if reduction occurs at that electrode with respect to the S.H.E and is given a negative sign, if oxidation occurs at the electrode with respect to the S.H.E.
EMF of cell and Electrode potential
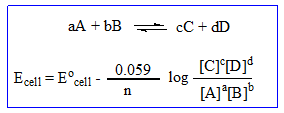
The electromotive force or EMF of a cell is expressed in term of potential difference established between the half cells when no current passes through the cell. Under standard condition of temperature and concentration EMF is known as standard EMF and abbreviated as E° . This is expressed in volts.

Nernst Equation
Dependence of electrode potential and EMF on concentration and temperature.
For electrode potential:
For EMF of a cell involving the redox reaction
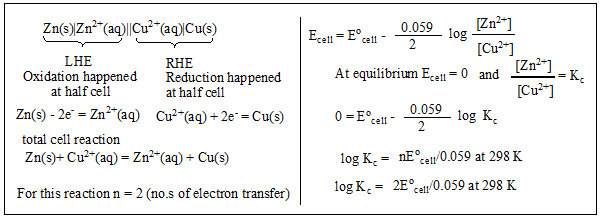
Q. What is the EMF of the following Denial cell? How can you calculate the equilibrium constant value for this cell?
Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s)
Some applications:
Purification of water: Ozone is a strong oxidizing agent and it is capable of destroying organic pollutants and bacteria present in water. Hence, oxidation reaction can be utilize for water purification process.
Electroplating: In this process electrical current is employed to reduce dissolved metal cation followed by deposition on a metal plate. This techniques is high in use to prevent corrosion in metals and its matalic machines.
Metallurgy: Metal ores are obtained in their complex form mainly as oxides, sulfides. Reduction of those ores provides pure metal, e.g. Hematite (Fe2O3), magnetite (Fe3O4) are reduced in the presence of carbon to have metallic iron.