Electrophilic Substitution Reactions
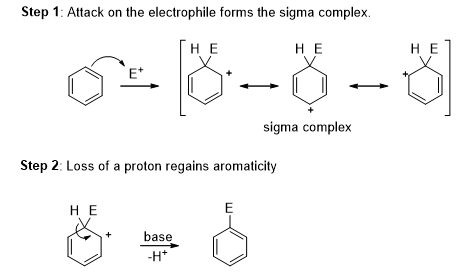
The π-bond electrons in benzenes attack a strong electrophile and lose its aromaticity to give a resonance stabilized carbocation, called a sigma complex. Loss of the proton on the tetrahedral carbon atom of the sigma complex helps to regain the aromaticity. The overall reaction is the electrophilic aromatic substitution reaction.
Halogenation
Halogens react with benzene in the presence of a strong Lewis acid such as AlCl3 or FeBr3 to give halobenzenes. For example, bromobenzene can be prepared with good yield as shown in below.
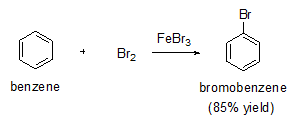
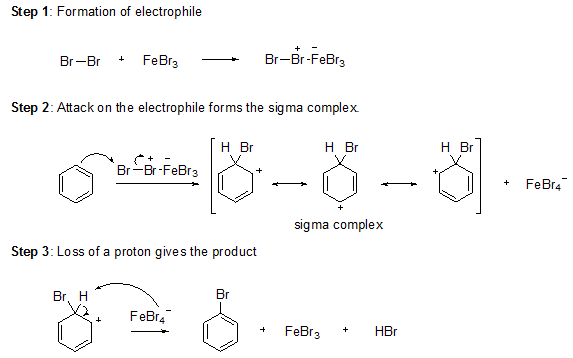
Bromine itself is not sufficiently electrophilic to react with benzene so that a strong Lewis acid such as FeBr3 used as a catalyst for the formation of Br+ which attacks benzene to form the sigma complex. Bromide ion acts as a weak base to remove a proton from the sigma complex, giving the substituted benzene and HBr.
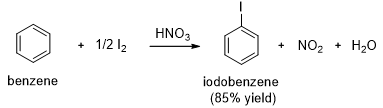
Chlorination of benzene works much like bromination. Aluminum chloride (A1Cl3) is often used as the Lewis acid catalyst for chlorination of benzene. Iodination of benzene requires an acidic oxidizing agent, such as nitric acid. The iodine cation, an electrophile, results from oxidation of iodine by nitric acid.
Nitration
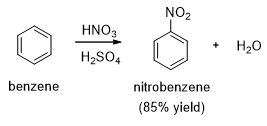
Nitration of benzene using a mixture of HNO3 and H2SO4 gives the target product rapidly at lower temperatures (Scheme 1).
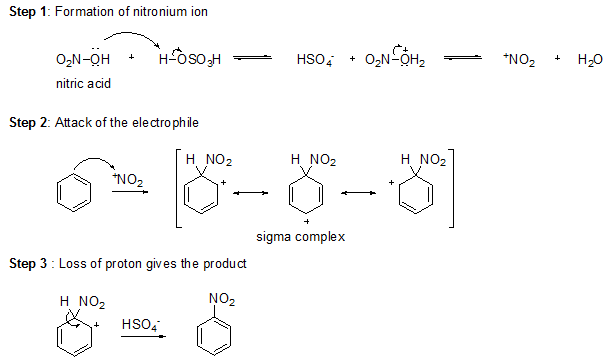
Sulfuric acid protonates the hydroxyl group of nitric acid, allowing it to leave as water and form a nitronium ion (+NO2), a powerful electrophile. The nitronium ion reacts with benzene to form a sigma complex. Loss of a proton from the sigma complex gives nitrobenzene.
Sulfonation
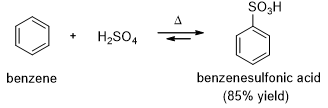
Aryl sulfonic acid can be easily synthesized by an electrophilic aromatic substitution using sulfur trioxide (SO3) as the electrophile (Scheme 3).
Sulfur trioxide is the anhydride of sulfuric acid. Although sulfur trioxide is uncharged, it is a strong electrophile where three sulfonyl (S=O) bonds drawing electron density away from the sulfur atom. Benzene attacks sulfur trioxide, forming a sigma complex. Loss of a proton on the tetrahedral carbon and reprotonation on oxygen gives benzenesulfonic acid.
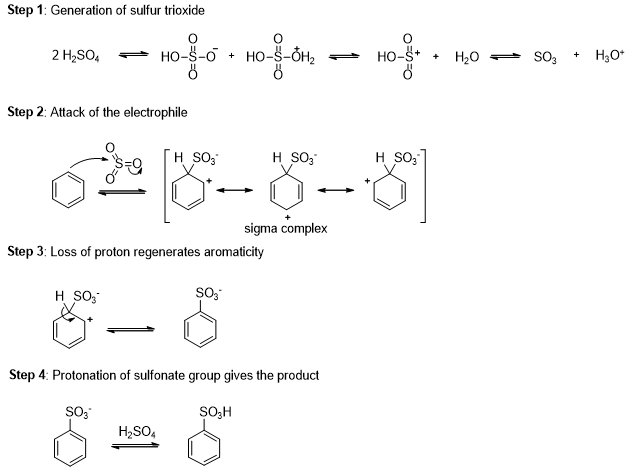
Sulfonation is reversible reaction, and a sulfonic acid group can be removed from an aromatic ring by heating in dilute sulfuric acid. Excess water removes SO3 from the equilibrium by hydrating it to sulfuric acid.
The Friedel-Crafts Alkylation
In the presence of Lewis acid catalysts such as aluminum chloride (AlCl3) or ferric chloride (FeCl3), alkyl halides react with benzene to give alkyl benzenes. This reaction is called the Friedel-Crafts alkylation.
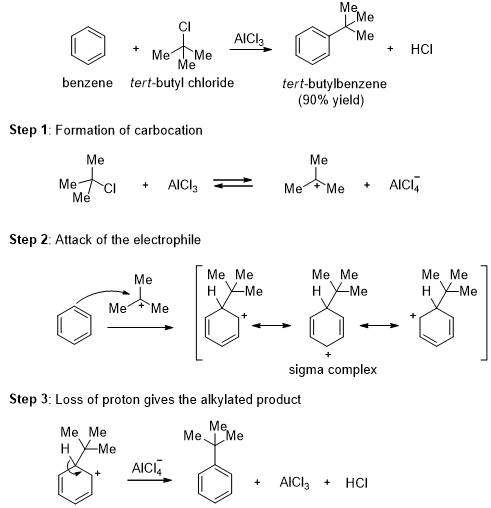
This alkylation is an electrophilic aromatic substitution reaction where the tert-butyl cation acts as the electrophile. The tert-butyl cation is formed by the reaction of tert-butyl chloride with the catalyst, aluminum chloride. The tert-butyl cation reacts with benzene to form a sigma complex. Loss of a proton gives the product. The aluminum chloride catalyst is regenerated in the final step.
The Friedel-Crafts Acylation
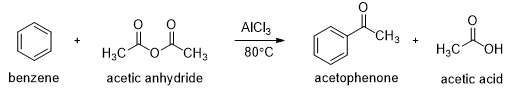
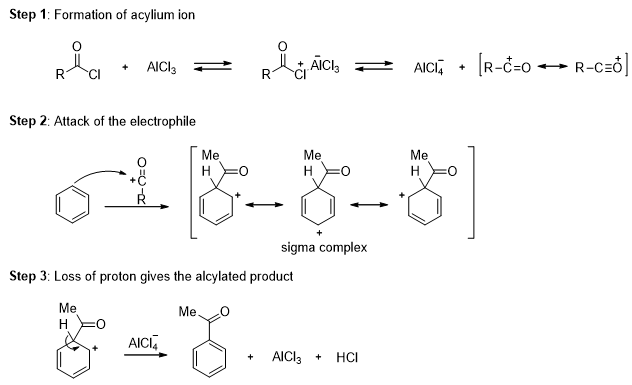
In the presence of aluminum chloride, an acyl chloride reacts with benzene to give acyl benzene. The Friedel-Crafts acylation is analogous to the Friedel-Crafts alkylation, except that the reagent is acyl chloride instead of an alkyl halide and the product is acyl benzene instead of alkyl benzene.
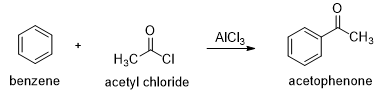
In the first step a resonance-stabilized acylium ion formed which reacts with benzene via an electrophilic aromatic substitution reaction to form an acyl benzene. The carbonyl group in the product has nonbonding electrons that can form a complex with the Lewis acid (AlCl3). Addition of water hydrolyzes this complex, giving the free acyl benzene. Friedel-Crafts reactions do not occur on strongly deactivated rings, so the acylation stops after one substitution.
Friedel-Crafts acylations can also be carried out using carboxylic acid anhydrides. For example, benzene reacts with acetic anhydride in the presence of Lewis acid to give acetophenone. Excess of benzene is used in this reaction to get good yield.