Electrophilic Substitution Reactions with Substituted Benzenes
Substituted benzenes undergo the electrophilic aromatic substitution reactions such as halogenation, nitration, sulfonation, alkylation and acylation. Some substituents make the ring more reactive and some make it less reactive than benzene toward electrophilic aromatic substitution. The rate determining step of an electrophilic aromatic substitution reaction is the formation of a carbocation intermediate. So substituents that are capable of donating electrons into the benzene ring can stabilize the carbocation intermediate, thereby increasing the rate of electrophilic aromatic substitution.
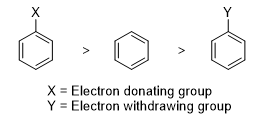
- In contrast, substituents that withdraw electrons from the benzene ring will destabilize the carbocation intermediate, thereby decreasing the rate of electrophilic aromatic substitution. The relative rates of electrophilic aromatic substitution reaction of benzene and substituted benzenes are given below.
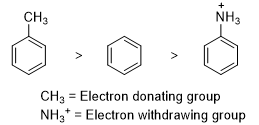
- Substituents can donate electrons into a benzene ring or can withdraw from benzene ring either by inductive effect or resonance effect. Alkyl substituents that are bonded to a benzene ring can donate electrons inductively. Donation of electrons through a σ-bond is called inductive electron donation. Withdrawal of electrons through a σ-bond is called inductive electron withdrawal. For example methyl group is an electron donating group because of hyperconjugation and NH3+ group is an electron withdrawing group because it is more electronegative than a hydrogen. The relative rates of electrophilic substitution decrease in the following order.
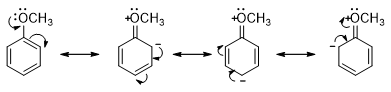
- Substituents such as OH, OR and Cl have a lone pair on the atom that is directly attached to the benzene ring. This lone pair can be delocalized into the ring. These substituents also withdraw electrons inductively because the atom attached to the benzene ring is more electronegative than a hydrogen. But electron donation into the ring by resonance is more significant than inductive electron withdrawal from the ring.
- Substituents such as C=O, C≡N and NO2 withdraw electrons by resonance. These substituents also withdraw electrons inductively because the atom attached to the benzene ring is more electronegative than a hydrogen.
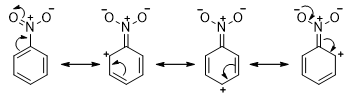
- Substituents that make the benzene ring more reactive toward electrophilic substitution, by donating electrons into the benzene ring, are called the activating groups. In contrast, substituents that make the benzene ring less reactive toward electrophilic substitution, by withdrawing electrons from the benzene ring, are called the deactivating groups.
- Strongly activating substituents such as -NH2, -NHR, -NR2 -OR, and –OH make the benzene ring more reactive toward electrophilic substitution. The moderately activating substituents such as –NHCOR and –OCOR, also donate electrons into the ring by resonance less effectively than that of strongly activating substituents. Alkyl, aryl, and -CH=CHR groups are weakly activating substituents.
- Strongly deactivating substituents such as -C≡N, -SO3H, -NO2, and ammonium ions make the benzene ring less reactive toward electrophilic substitution. Carbonyl compounds are moderately deactivating substituents and the halogens are weakly deactivating substituents.
- Substituted benzene undergoes an electrophilic substitution reaction to give an ortho-isomer, a meta-isomer, a para-isomer or mixture of these isomers. The substituent already attached to the benzene ring determines the location of the new substituent.
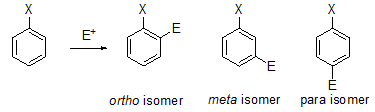
-
- All activating substituents and weakly deactivating halogens are ortho–para directors, and all substituents that are more deactivating are meta directors. When substituted benzene undergoes an electrophilic substitution reaction, an ortho-substituted carbocation, a meta-substituted carbocation, and a para-substituted carbocation can be formed. The relative stabilities of the three carbocations determine the preferred pathway of the reaction.
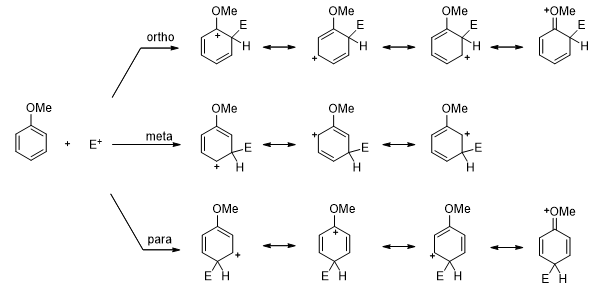
- The methoxy substituent (an activating group), for example, donates electron into the ring and stabilize the ortho– and para-substituted carbocations as shown. Therefore, the most stable carbocation is obtained by directing the incoming group to the ortho and para positions. Thus, any substituent that donates electrons is an ortho-para director.
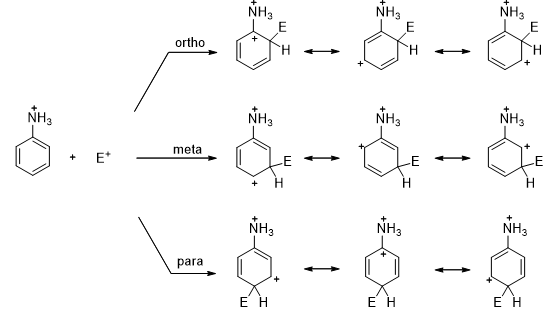
- In contrast, the ammonuium ion substituent (a deactivating group), for example, withdraws electron from the ring and destabilize the ortho– and para-substituted carbocations as shown. Therefore, the most stable carbocation is obtained by directing the incoming group to the meta position. Thus, any substituent that withdraws electrons is a meta director.
- In the following examples, the methoxy group and ethyl group are activating substituents which preferably direct the incoming electrophile to ortho and para position. These substituted benzenes undergo electrophilic aromatic substitution faster than benzene.
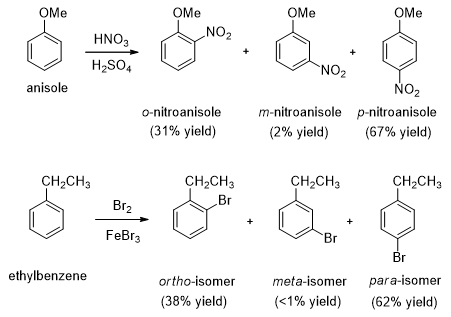
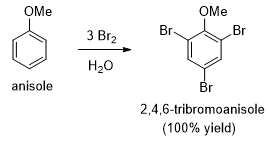
- A methoxy group is so strongly activating group so that anisole quickly brominates in water without a catalyst. In the presence of excess bromine, this reaction proceeds to give the tribromide substituted product.