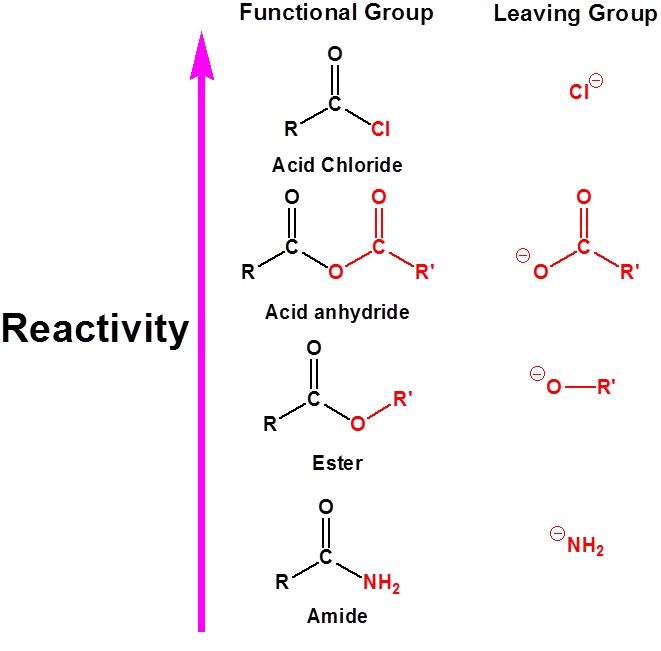
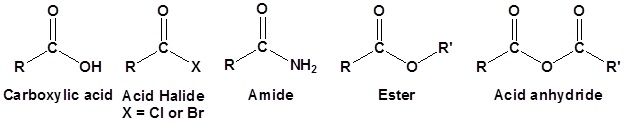
The chemistry of Carboxylic acid derivatives functional groups is closely related. The main difference is the presence of an electronegative substituent that can act as a leaving group during nucleophile substitution reactions. Although there are many types of carboxylic acid derivatives known we will be focusing on just four:
- Acid halides,
- Acid anhydrides,
- Esters, and
- Amides.
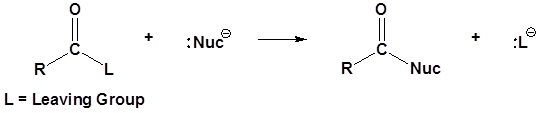
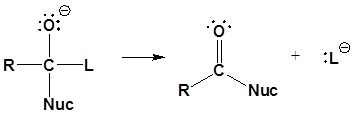
General reaction
General mechanism
1) Nucleophilic attack on the carbonyl
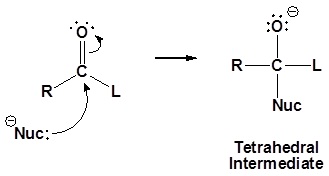
2) Leaving group is removed
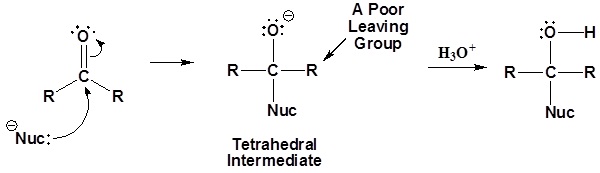
Although aldehydes and ketones also contain a carbonyl their chemistry is distinctly different because they do not contain a suitable leaving group. Once the tetrahedral intermediate is formed aldehydes and ketones cannot reform the carbonyl. Because of this aldehydes and ketones typically undergo nucleophilic additions and not substitutions.
The relative reactivity of carboxylic acid derivatives toward nucleophile substitutions is related to the electronegative leaving group’s ability to activate the carbonyl. The more electronegative leaving groups withdrawn electron density from the carbonyl, thereby, increasing its electrophilicity.