Nitrogen
Lack of reactivity
- You know that nitrogen is an unreactive gas because it is mixed with reactive oxygen in Earth’s atmosphere and reacts very little.
- The reason for this lack of reactivity is the very strong N≡N bond in the molecule. The two nitrogen atoms share three electron pairs, which form a triple covalent bond, and each atom retains a lone pair of electrons.
- The bond energy for nitrogen is 944 kJ mol−1 compared with the bond energy in fluorine, F2, which is 158 kJ mol−1.

Structure of nitrogen
Although nitrogen is unreactive, it does react under the right conditions. For example, burning magnesium reacts with the nitrogen in air to form magnesium nitride:
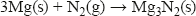
At high temperatures, nitrogen reacts with oxygen to form oxides of nitrogen, for example:

In car engines (and in thunderstorms) nitrogen combines with oxygen to produce a mixture of oxides of nitrogen often referred to as NOx.
Nitrogen also reacts with hydrogen to form ammonia:

This is the basic reaction in the Haber process for the manufacture of ammonia.
Finally, the roots of some plants of the pea and bean family have nodules that contain bacteria able to ‘fix’ nitrogen chemically. The bacteria convert the nitrogen into ammonium ions, which plants can use to make proteins.
Ammonia
Ammonia, NH3, is an alkaline gas. You might expect it to have a trigonal planar shape, but it is pyramidal with a lone pair of electrons occupying the apex,

Structure of ammonia
Remember that a lone pair takes up more space than a pair of bonding electrons. In the case of ammonia this reduces the H–N–H bond angles to about 107°. The relatively high electronegativity (3.0) of nitrogen means that ammonia can form hydrogen bonds, and as a result it is very soluble in water.
Ammonia is also a base, accepting a proton to form the ammonium ion, NH4+:

This ion is tetrahedral, the proton forming a coordinate (dative) bond with the nitrogen atom using the lone pair
In the laboratory, ammonia is easily displaced from ammonium compounds by warming with a strong base such as sodium hydroxide:
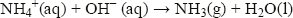

Structure of the ammonium ion
The uses of ammonia and its compounds
Ammonia is one of the most important bulk chemicals manufactured, mainly because of its use in the production of fertilisers. In 2019, more than 235 million tonnes of ammonia were produced worldwide.
As well as its use in fertiliser manufacture (mainly as the sulfate or nitrate), ammonia can be oxidised using a platinum catalyst to form nitrogen monoxide:
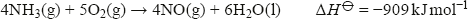
This exothermic reaction is the starting point for the manufacture of nitric acid. The hot gas is cooled, reacted with more oxygen to form nitrogen dioxide, and then dissolved in water to form nitric acid:
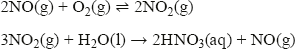
Nitrogen compounds and pollution
Nitrates
Nitrogen compounds, particularly nitrates such as NH4NO3, are used to make fertilisers. These have had significant benefits in increasing crop yields around the world. However, if excessive amounts of fertilisers are used, they can get washed into streams and rivers and this can have two effects:
• The nitrates can get into drinking water supplies, from which they are difficult to remove. They can affect the ability of babies under 6 months to carry oxygen in the bloodstream.
• They can increase the growth of aquatic vegetation, which then decays reducing oxygen levels in streams and rivers, affecting other forms of aquatic life.
Nitrogen oxides
- The combustion of motor fuels generates temperatures high enough to form oxides of nitrogen, NO and NO2, in exhaust gases.
- These gases react, in the presence of strong sunlight, with unburnt hydrocarbons in the lower atmosphere to form peroxyacetyl nitrate, PAN. This is a component of photochemical smog and is known to contribute to respiratory problems. It is especially dangerous to sufferers of asthma and at higher concentrations can cause crop damage, reducing yields.
- These gases also have a polluting effect in the upper atmosphere, where they catalyse the oxidation of sulfur dioxide. The main source of sulfur dioxide in the atmosphere is the combustion of fuels (mainly coal and oil) that contain sulfur or its compounds. Some of the sulfur dioxide is removed from the waste gases emitted by major users of these fuels, such as power stations.
- The exact mechanism is uncertain, but the following is a possibility:

- The emission of nitrogen oxides from vehicles has reduced greatly since the introduction of catalytic converters. These also reduce the emission of carbon monoxide and unburnt hydrocarbons

Sulfur
Sulfur dioxide and acid rain
- the combustion of sulfur-containing fuels releases sulfur dioxide into the atmosphere, and that in the presence of oxides of nitrogen this can be converted into sulfuric acid.
- This decreases the pH of rain. Acid rain can harm plants and animals both directly and indirectly by making lakes acidic.
- It also releases toxic metals such as aluminium from soils, and below pH 4.5 no fish are likely to survive. This has effects further up the food chain.
- Acid rain also increases the erosion of limestone-based buildings and statues.
The production and some effects of acid rain are shown in.

Practice Questions
- explain the lack of reactivity of nitrogen
- describe and explain the basicity of ammonia, the structure of the ammonium ion and the displacement of ammonia from ammonium salts
- state and explain the natural and man-made occurrences of oxides of nitrogen and their catalytic removal from vehicle exhausts
- describe the role of oxides of nitrogen in the formation of acid rain both directly and in the oxidation of atmospheric sulfur dioxide