Nitrogen Compounds
|
Primary Amine |
Secondary Amine |
Tertiary Amine |
|
|
|
|
Formation of Alkyl Amines
Substitution
- R–X + NH3 → R–NH2 + HX(g)
-
Type of reaction:
- nucleophilic substitution
-
Reagent:
- hot conc. NH3 in ethanol
-
Condition:
- heat under reflux
|
Reduction |
|
|
R–C ≡ N + 4[H] → R–CH2NH2 |
R–CO–NH2 + 4[H] → R–CH2NH2 + H2O |
|
Type of reaction: reduction of a nitrile |
Type of reaction: reduction of an amide |
|
Reagent: hydrogen gas |
Reagent: hydrogen gas |
|
Reducing Agents (catalyst): LiAlH4 in dry ether Nickel |
Reducing Agent (catalyst): LiAlH4 in dry ether |
|
Conditions: high temp. and pressure |
Conditions: r.t.p. |
Formation of Aryl Amines
Reduction

-
Type of reaction:
- reduction of nitrobenzene
-
Reagent:
- conc. HCl with Tin
-
Condition:
- Heat
Basicity of Amines
- Bases are proton acceptors (electron donors)
- N-atoms in amines have a lone pair of e–s
- N donates lone pair and accepts H+ forming dative bond

Relative Basicity

|
Phenyl amine weaker than ammonia |
Ammonia |
Ethylamine stronger than ammonia |
|
|
|
|
||
|
Lone pair of e–s on N gets partially delocalized by interaction with benzene e– cloud |
Ethyl e– donating group, increases e– density on N |
||
|
Lone pair less available for coordination to proton |
Enhanced ability to donate lone pair of e–s to proton |
||
|
Alkyl ammonium cation formed more stable than NH4+ cation from ammonia |
|||
Reactions of phenyl amine
Bromination

-
Type of reaction:
- electrophilic substitution
-
Reagent:
- aq. Bromine
-
Conditions:
- r.t.p. (no catalyst)
- Forms a white ppt.
Diazotization

(–N2+ is called diazonium ion)
-
Type of reaction:
- diazotization reaction
-
Reagent:
- nitrous acid (NaNO2 with excess HCl(aq))
- (HNO2 weak, unstable acid so produced by reaction NaNO2 with excess HCl)
-
Condition:
- low temp. (5
0C)
- low temp. (5
-
Note:
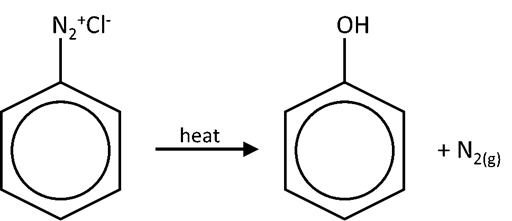
- Diazonium salts of aryl amine stabilized by delocalization of –N2+ ion’s e–s over benzene ring
- However, diazonium ion highly unstable and can decompose above 10oC to
Coupling Reactions of Diazonium Salts
- Type of reaction: electrophilic substitution
- Reagent: aromatic amines or phenols
- Azo compounds are complex compounds involving a minimum of two aromatic rings joined by N=N coupling
- Benzene diazonium ion carries a +ve charge and readily reacts with cold alkaline solutions of aromatic amines and phenols to give brightly coloured azo-compounds

Bright orange dye formed
-
By using alterative aryl compounds to phenol, a range of brightly coloured dye can be formed.
Formation of Amides
Formation
- Primary Amides:

- Secondary Amides:

-
Type of reaction:
- nucleophilic substitution
-
Reagent:
- conc. NH3(aq)
-
Condition:
- r.t.p.
- Example:

ethanoyl chloride + methyl amine → N-methyl methanamide
Neutrality of Amides
- The presence of the electron withdrawing oxygen atom means that the lone pair on the amide’s nitrogen atom is not available to be donated to e.g. H+ ions
- Hence, amides are neutral

Hydrolysis of Amides

Amino Acids

- Optical activity: all amino acids (except glycine) have a chiral carbon therefore they are optically active
Acid/Base Properties of Amino Acids
-
Basic amino group and acidic carboxyl group interact:
- Carboxyl group donates a proton to amino group
- Amino group accepts proton and zwitterion formed

- Zwitterion: ion that contains regions of +ve & –ve charge
- Amino acids solids at r.t.p. due to ionic bonds that exist between zwitterions
- Presence of zwitterions means that amino acids are soluble in water
Amino Acids in Acidic/Basic Conditions
- If acid added, the –COO– part of the zwitterion accepts an H+ ion, reforming –COOH group, leaving +ve charge
- If alkali added, the –NH3+ part of the zwitterion donates an H+ ion to the OH–, reforming –NH2 group and H2O, leaving a -ve charge

Peptide Bonds
- Amide link formed by nucleophilic attack of –NH2 group of one amino acid on –COOH group of another

- Reaction is a condensation reaction as H2O eliminated
- Reaction can continue to occur as product still has –NH2 and –COOH group present
Dipeptide → Tripeptide → Polypeptide (protein)
- Proteins are polymers of amino acids; many polypeptide chains held together by intermolecular forces
- Hydrolysis: involves breaking of peptide links by reaction with water catalysed by an acid or alkali catalyst, giving back the amino acids, temp. nearly 90oC
Electrophoresis
- It is used to separate, identify and purify amino acids obtained when protein hydrolysed
-
Technique based on separating ions placed in an electric field. When sample placed between two electrodes:
- +ve charge ions move towards –ve charged electrode
- –ve charge ions move towards +ve charged electrode

- Sample placed on absorbent filter paper (or gel)
- Buffer solution carries ions along (back or forth)
|
Factors that Determine the |
|
|
Direction of Travel |
Speed of Movement |
|
pH of buffer solution Charge on amino acid |
Voltage applied Temperature Size (Mr) of amino acid Magnitude of charge |
|
Larger ions with longer side chain move slower More highly charge ions move faster |
|




