Nucleophilic Substitution of Benzenes
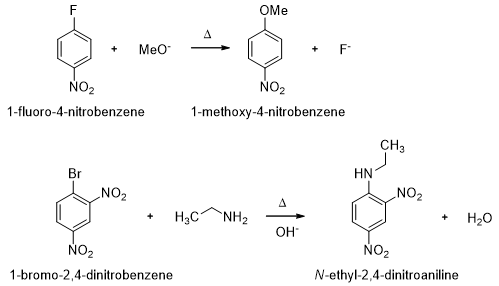
Aryl halides do not react with nucleophiles under the standard reaction conditions because the electron clouds of aryl ring repel the approach of a nucleophile. Nucleophiles can displace halide ions from aryl halides, if there are strong electron-withdrawing groups ortho or para to the halide. This class of reactions is called nucleophilic aromatic substitution reaction..
-
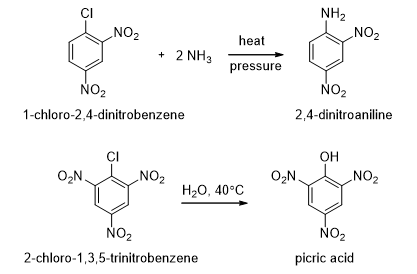
- Electron-withdrawing substituents such as nitro group make the ring reactive towards nucleophilic aromatic substitution but without at least one powerful electron-withdrawing group, the nucleophilic aromatic substitutions would be difficult. The mechanism of nucleophilic aromatic substitution cannot be the SN2 mechanism because aryl halides cannot achieve the correct geometry for back-side approach of a nucleophile. The SN1 mechanism also cannot be involved.
- Consider the reaction of 2,4-dinitrochlorobenzene with a nucleophile (Scheme 8). When a nucleophile attacks the carbon bearing the chlorine, a negatively charged sigma complex results. The negative charge is delocalized over the ortho and para carbons of the ring and further delocalized into the electron-withdrawing nitro groups. Loss of chloride from the sigma complex gives the nucleophilic substituted product.
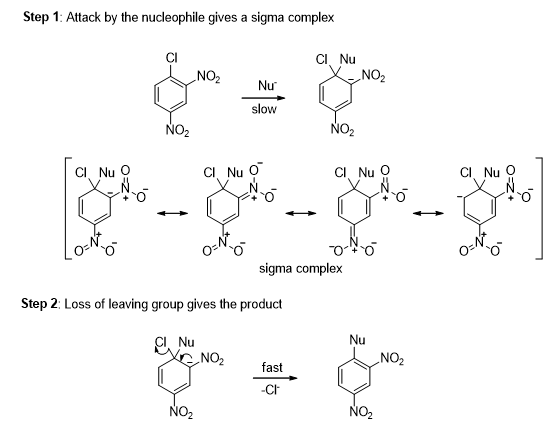
- The leaving group ability of halogen in nucleophilic aromatic substitution reaction is following the order: F > Cl > Br > I. The incoming group should be a stronger base than the group that is being replaced.