What are polyamides?
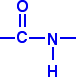
Polyamides are polymers where the repeating units are held together by amide links. An amide group has the formula – CONH2. An amide link has this structure:
Nylon
In nylon, the repeating units contain chains of carbon atoms. (That is different from Kevlar, where the repeating units contain benzene rings) There are various different types of nylon depending on the nature of those chains.
Nylon-6,6
Nylon-6,6 is made from two monomers each of which contain 6 carbon atoms – hence its name. One of the monomers is a 6 carbon acid with a -COOH group at each end – hexanedioic acid. The other monomer is a 6 carbon chain with an amino group, -NH2, at each end. This is 1,6-diaminohexane
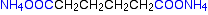
When these two compounds polymerize, the amine and acid groups combine, each time with the loss of a molecule of water. This is known as condensation polymerization. Condensation polymerization is the formation of a polymer involving the loss of a small molecule. In this case, the molecule is water, but in other cases different small molecules might be lost.
The diagram shows the loss of water between two of the monomers:
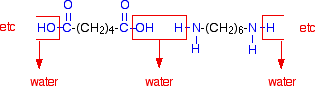
This keeps on happening, and so you get a chain which looks like this:
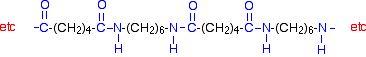
Nylon-6
it is possible to get a polyamide from a single monomer. Nylon-6 is made from a monomer called caprolactam.
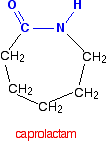
Notice that this already contains an amide link. When this molecule polymerizes, the ring opens, and the molecules join up in a continuous chain.
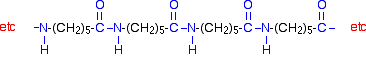
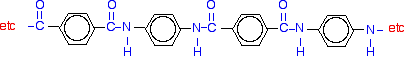
Kevlar
Kevlar is similar in structure to nylon-6,6 except that instead of the amide links joining chains of carbon atoms together, they join benzene rings. The two monomers are benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene.
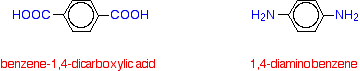
If you line these up and remove water between the -COOH and -NH2 groups in the same way as we did with nylon-6,6, you get the structure of Kevlar: