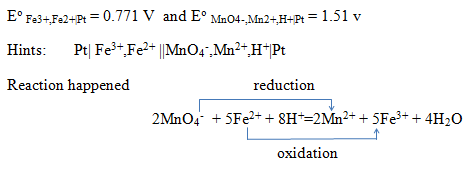The reactions which involve oxidation & reduction are called redox reaction. e.g .
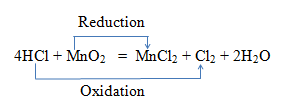
In here HCl has been oxidised to Cl2 and MnO2 has been reduced to MnCl2 .
Oxidation number
It’s the charge which an atom of an element has in its ion or appear to have when present in the combined state. It is also known as oxidation state.
Rules for calculation of oxidation number:
- Oxidation number of any atom in the elementary state is zero.
e . g . in O2 , H2 , Na, He and Fe the oxidation state of each atom equal to zero.
- Oxidation number of mono atomic ion is equal to the charge on it.
- Oxidation number of H is +1 when combined with non metal and -1 when combined with active metal like Na, Ca etc. e . g . NaH, CaH2 .
- Oxidation number of oxygen is -2 except in peroxides like H2O2 , Na2O2 etc where it is -1 and OF2 where it is +2.
- Oxidation number of alkali and alkaline earth metals is +1 and +2 respectively.
- Oxidation number of halogens is in -1 in metal halides.
- In compounds of metal with non-metal, metal have positive oxidation numbers whereas non-metals have negative Oxidation numbers.
- In compound of two different elements, the more electronegative has negative Oxidation number and the other has positive oxidation number.
- In complex ions, the sum of the oxidation numbers of all atoms is equal to the charge on the ion.
Oxidation (de-electronation): loss of electron or result in the increase in oxidation number of its atom/s.
Oxidising agent
acceptor of electron/s.
Reduction (Electronation): Gain of electron/s or decrease in oxidation number of its atom/s.
Reducing agent: Donor of electron/s.
Redox potential
Any oxidation -reduction (redox) reaction can be divided into two half reactions: one in which a chemical species undergoes oxidation and one in which another chemical species undergoes reduction. If a half- reaction is written as a reduction, the driving force is the reduction potential. If the half-reaction is written as oxidation, the driving force is the oxidation potential related to the reduction potential by a sign change. So the redox potential is the reduction/ oxidation potential of a compound measured under standards conditions against a standard reference half-cell.
In biological systems the standard redox potential is defined at pH – 7.0 versus the hydrogen electrode and partial pressure of hydrogen = 1 bar .
| e . g . | 2Ag + Cl2 = 2AgCl |
The above redox reaction can be split into two half reactions
2 Ag = 2Ag+ + 2e or Ag = Ag+ + e – – – – – – – – – – – – – – – – – – – – – – – – – (I)
Cl2 + 2e = 2Cl− or ½ Cl2 + e = Cl− – – – – – – – – – – – – – – – – – – – – – – – – – – (II)
Reaction (I) is a oxidation process & the potential of this reaction is oxidation potential whereas potential of reaction (II) is reduction potential.
Analysis of redox cycle:
The half-cell potential of two half cells is given below. What is the redox reaction happened when they are combined in a favourable condition?