The Valence Shell Electron Repulsion (VSEPR) model
- can predict the structure of most molecules and polyatomic ions in which the central atom is a nonmetal;
- it also works for some structures in which the central atom is a metal.
- pairs of electrons (in bonds and in lone pairs) repel each other.The pairs of electrons (in bonds and in lone pairs) are called “groups”.
- Because electrons repel each other electrostatically, the most stable arrangement of electron groups (i.e., the one with the lowest energy) is the one that minimizes repulsion.
- Groups are positioned around the central atom in a way that produces the molecular structure with the lowest energy.
- it accurately predicts the three-dimensional structures of a large number of compounds.
We can use the VSEPR model to predict the geometry around the atoms in a polyatomic molecule or ion by focusing on the number of electron pairs (groups) around a central atom of interest. Groups include bonded and unbonded electrons; a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron each count as one group. The molecule or polyatomic ion is given an AXmEn designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group (usually a lone pair of electrons), and m and n are integers. The number of groups is equal to the sum of m and n. We can describe the molecular geometry around a central atom, that is, the arrangement of the bonded atoms in a molecule or polyatomic ion.
The geometries that are predicted from VSEPR when a central atom has only bonded groups (n = 0) are listed below
|
Groups around central atom (m + n) |
Geometry Name | Geometry Sketch | Predicted bond Angle | Example |
|---|---|---|---|---|
| 2 | linear | 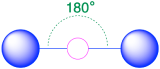 |
180° | 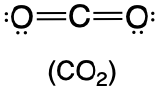 |
| 3 | trigonal plane | 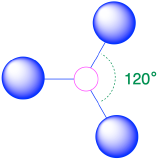 |
120° | 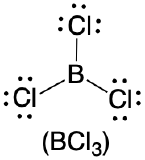 |
| 4 | tetrahedron | 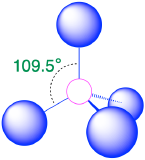 |
109.5° | 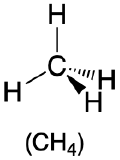 |
| 5 | trigonal bipyramid | 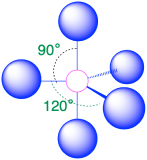 |
90° and 120° | 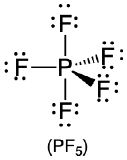 |
| 6 | octahedron | 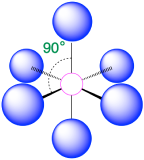 |
90° | 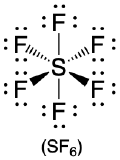 |
| 7 | pentagonal bipyramid | 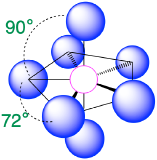 |
90° and 72° |  |
| 8 | square antiprism | 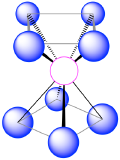 |
70.5°, 99.6° and 109.5° | 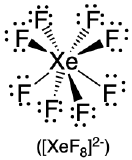 |