The Periodic Table: chemical periodicity
This section on the Periodic Table and chemical periodicity builds on work covered in the first three chapters of this guide. In this chapter we will concentrate on Period 3 – the elements sodium to argon.
Physical properties of Period 3 elements
You need to know and be able to explain the variation of four key physical properties across the third period (sodium to argon). These are:
- atomic and ionic radius
- melting point
- electrical conductivity
- ionisation energy
Atomic and ionic radius
The Periodic Table is an arrangement of the chemical elements according to their proton numbers. The word ‘periodic’ suggests a regular recurrence of a feature. Look at the graph of atomic volume (which is linked directly to atomic radius) shown

Relationship between atomic volume and atomic number
- A pattern is apparent – the Group 1 metals occur at peaks on the graph. The other thing that is noticeable is that atoms of the Group 1 metals get larger moving down the group.
- Moving across Period 3 from left to right, the ionic radii change (Figure 9.2). To begin with, elements lose electrons to form positive ions. The increasing positive charge pulls the remaining electrons closer to the nucleus to give a smaller ionic radius.
- Beyond silicon, Si4+, the atoms form negative ions with a decreasing charge. The electrons gained fill the outer electron shell. This means that the ionic radius is much larger for phosphorus, P3−, and then decreases steadily to chlorine, Cl–.

Melting point
Period 3 contains different types of elements, from metals on the left-hand side through non-metallic solids to gases on the right-hand side. The melting points and boiling points of these elements are shown in Figure 9.3.
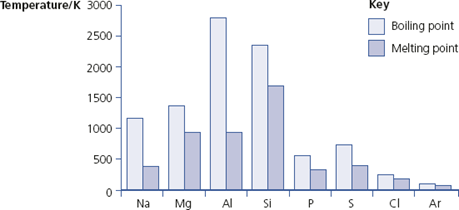
Melting points and boiling points of Period 3 elements
• The elements sodium, magnesium and aluminium are metals and their atoms are bonded using a ‘sea’ of delocalised electrons. The melting (and boiling) points increase because the number of electrons each atom contributes to the ‘sea’ increases.
• Silicon is a semi-conductor with a giant covalent structure (similar to that of diamond), so it has a high melting point.
• Phosphorus is a non-metal with four atoms in its molecules. To melt it, only van der Waals’ forces have to be overcome, so phosphorus has a low melting point.
• Sulfur, another non-metal, is made up of S8 molecules. Because the molecules are big, there are stronger van der Waals’ forces between them than in phosphorus. So sulfur has a higher melting point than phosphorus.
• A chlorine molecule, Cl2, has only two atoms so the melting point is lower than that of sulfur.
• Finally, argon consists of single atoms with very weak van der Waals’ forces so argon has the lowest melting (and boiling) point in Period 3.
Electrical conductivity
Sodium, magnesium and aluminium are good electrical conductors because of the ‘sea’ of delocalised electrons they have. Silicon is a semi-conductor, but not as good a conductor as graphite. All the other elements are electrical insulators.
First ionisation energy
The changes in first ionisation energy across Period 3.
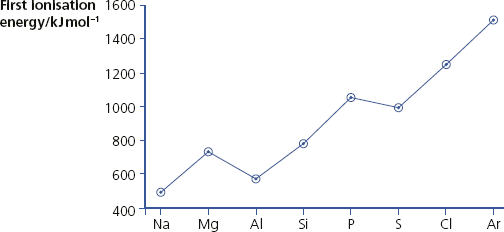
Changes in first ionisation energy across Period 3
There are four things that affect the size of the first ionisation energy:
• the charge on the nucleus
• the distance of the electron from the nucleus
• the number of electrons between the outer electrons and the nucleus
• whether the electron to be removed is alone or paired
Across Period 3, the charge on the nucleus increases by one unit for each element. In all cases, electrons are being removed from the third shell and these are screened by the 1s2, 2s2 and 2p6 electrons. The graph is not a straight line because of the orbitals the electrons are removed from.
The first ionisation energy of aluminium is lower than that of magnesium. This fall is because in aluminium the electron being removed is in a p-orbital and is, on average, further away from the nucleus than an electron in an s-orbital.
There is another drop between phosphorus and sulfur – this is caused by removing a paired electron in a p-orbital. The repulsion between these two electrons makes it easier to remove one of them than a single electron in a p-orbital
Chemical properties of Period 3 elements
You have to be able to recall the reactions of these elements with oxygen, chlorine and water, and to know about the reactions of any oxides and chlorides formed.
Reactions with oxygen
| Element | Reaction | Product(s) | Equation |
| Sodium | Burns with an orange-yellow flame to give white products | Sodium oxide and peroxide | 4Na + O2 → 2Na2O 2Na + O2 → Na2O2 |
| Magnesium | Burns with a bright white flame to give a white product | Magnesium oxide | 2Mg + O2 → 2MgO |
| Aluminium | Powder burns to give a white product | Aluminium oxide | 4Al + 3O2 → 2Al2O3 |
Chemical properties of Period 3 elements
You have to be able to recall the reactions of these elements with oxygen, chlorine and water, and to know about the reactions of any oxides and chlorides formed.
Reactions with oxygen
| Element | Reaction | Product(s) | Equation |
| Sodium | Burns with an orange-yellow flame to give white products | Sodium oxide and peroxide | 4Na + O2 → 2Na2O 2Na + O2 → Na2O2 |
| Magnesium | Burns with a bright white flame to give a white product | Magnesium oxide | 2Mg + O2 → 2MgO |
| Aluminium | Powder burns to give a white product | Aluminium oxide | 4Al + 3O2 → 2Al2O3 |
| Silicon | Burns if heated strongly | Silicon dioxide | Si + O2 → SiO2 |
| Phosphorus | Burns with a yellow flame producing clouds of white smoke | Phosphorus(III) oxide; phosphorus(V) oxide in excess O2 | P4 + 3O2 → P4O6 P4 + 5O2 → P4O10 |
| Sulfur | Burns with a blue flame producing a colourless gas | Sulfur dioxide (sulfur trioxide is produced in the presence of a catalyst and excess O2) | S + O2 → SO2 |
| Chlorine | Does not react directly with oxygen | ||
| Argon | No reaction |
Reactions with chlorine
You may not have seen as many of the elements reacting with chlorine. The reactions are summarised
| Element | Reaction | Product | Equation |
| Sodium | Burns with a bright orange flame giving a white product | Sodium chloride | 2Na + Cl2 → 2NaCl |
| Magnesium | Burns with a bright white flame giving a white product | Magnesium chloride | Mg + Cl2 → MgCl2 |
| Aluminium | Burns with a yellow flame giving a pale-yellow product | Aluminium chloride | 2Al + 3Cl2 → 2AlCl3 |
| Silicon | Reacts when chlorine gas is passed over it to form a colourless liquid | Silicon tetrachloride | Si + 2Cl2 → SiCl4 |
| Phosphorus | Burns with a yellow flame to form a mixture of chlorides | Phosphorus(III) chloride and phosphorus(V) chloride | P4 + 6Cl2 → 4PCl3 P4 + 10Cl2 → 4PCl5 |
| Sulfur | Reacts when chlorine gas is passed over it to form an orange liquid | Disulfur dichloride | 2S + Cl2 → S2Cl2 |
| Chlorine | No reaction | ||
| Argon | No reaction |
Reactions with water
Sodium reacts violently with water, releasing hydrogen gas and dissolving to form sodium hydroxide solution:

Magnesium reacts slowly with cold water, forming magnesium hydroxide and hydrogen:

It reacts vigorously if steam is passed over the heated metal, forming magnesium oxide and hydrogen:

Oxidation numbers in oxides and chlorides
| Aluminium | No reaction | ||
| Silicon | No reaction | ||
| Phosphorus | Phosphorus(III) oxide reacts with cold water | 1–2 | P4O6 + 6H2O → 4H3PO3 |
| Phosphorus(V) oxide reacts violently | 1–2 | P4O10 + 6H2O → 4H3PO4 | |
| Sulfur | Sulfur dioxide dissolves readily | 1 | SO2 + H2O → H2SO3 |
| Sulfur trioxide reacts violently | 0 | SO3 + H2O → H2SO4 | |
| Chlorine | Does not react directly with oxygen | ||
| Argon | No reaction |
Aluminium oxide is amphoteric. This means that it reacts with both acids and alkalis. Aluminium oxide contains oxide ions, so it reacts as a base with acids, in a similar way to magnesium oxide:

It also has significantly acidic tendency, reacting with alkalis, such as sodium hydroxide, to form an aluminate:


Oxidation numbers of the oxides and chlorides of Period 3 elements
• The first four elements have positive oxidation numbers that correspond to the loss of all their outer electrons (silicon can also gain four electrons in forming its hydride, SiH4 – its oxidation number is still +4).
• Elements in Groups 15, 16 and 17 can also show positive oxidation numbers in their oxides and chlorides. You may think that it is unusual for non-metals to have positive oxidation numbers, but carbon has a positive oxidation number in carbon dioxide.
Reactions of oxides with water and their acid–base behaviour
The general trend is that alkalis are formed on the left-hand side of the period, aluminium and silicon oxides are almost insoluble, and acids are formed on the right-hand side.
| Oxide | Reaction | pH of solution | Equation |
| Sodium | Dissolves exothermically | 14 | Na2O + H2O → 2NaOH |
| Magnesium | Slight reaction | 09 | MgO + 2H2O → Mg(OH)2 |
| Aluminium | No reaction | ||
| Silicon | No reaction | ||
| Phosphorus | Phosphorus(III) oxide reacts with cold water | 1–2 | P4O6 + 6H2O → 4H3PO3 |
| Phosphorus(V) oxide reacts violently | 1–2 | P4O10 + 6H2O → 4H3PO4 | |
| Sulfur | Sulfur dioxide dissolves readily | 1 | SO2 + H2O → H2SO3 |
| Sulfur trioxide reacts violently | 0 | SO3 + H2O → H2SO4 | |
| Chlorine | Does not react directly with oxygen | ||
| Argon | No reaction |
Aluminium oxide is amphoteric. This means that it reacts with both acids and alkalis. Aluminium oxide contains oxide ions, so it reacts as a base with acids, in a similar way to magnesium oxide:
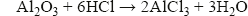
It also has significantly acidic tendency, reacting with alkalis, such as sodium hydroxide, to form an aluminate:

Reactions of the chlorides with water
The reactions of the chlorides of Period 3 elements with water give clues about the bonding present.
This is linked to the electronegativity of the element. the bigger the difference in electronegativity, the more polar is the bond. Sodium and chlorine have a difference of 2.1 whereas sulfur and chlorine have a difference of only 0.5.
| Chloride | Bonding | Electronegativity | Reaction | Equation |
| Sodium | Ionic (electrovalent) | 0.9 | Dissolves to give Na+ and Cl− ions | |
| Magnesium | Ionic (electrovalent) | 1.2 | Dissolves to give Mg2+ and Cl− ions | |
| Aluminium | Mainly covalent | 1.5 | Hydrolyses | AlCl3 + 3H2O → Al(OH)3 + 3HCl |
| Silicon | Covalent | 1.8 | Hydrolyses | SiCl4 + 2H2O → SiO2 + 4HCl |
| Phosphorus | Covalent | 2.1 | Hydrolyses | PCl3 + 3H2O → H3PO3 + 3HCl |
| Sulfur | Covalent | 2.5 | Hydrolyses | 2S2Cl2 + 2H2O → SO2 + 4HCl + 3S |
| Chlorine | No reaction | 3.0 | ||
| Argon | No reaction |
Bonding and electronegativity
In studying the trends in behaviour described above we need to consider the reasons for the trends. In order to make sense of these we think about the bonds being formed in the compounds and the electronegativity of the elements concerned.
As we go from left to right across the period, the bonding in both the oxides and the chlorides changes from ionic to covalent. Electronegativity increases across the period and thus the difference in electronegativity between the element and oxygen or the element and chlorine decreases.
| Element | Na | Mg | Al | Si | P | S | Cl |
| Electronegativity | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 |