Basic structure:
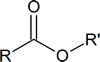
Examples of esters
| IUPAC name | R | R′ |
|---|---|---|
| octyl ethanoate | CH3 | CH3(CH2)6CH2 |
| propyl ethanoate | CH3 | CH3CH2 CH2 |
| 2‑methylpropyl propanoate | CH3CH2 | (CH3)2CHCH2 |
| methyl butanoate | CH3CH2CH2 | CH3 |
| ethyl butanoate | CH3CH2CH2 | CH3CH2 |
Preparation of Esters
- nucleophilic acyl substitution of an acid chloride with an alcohol.
- Acid ahydrides and carboxylic acids can react with alcohols to form esters.
- deprotonating a carboxylic acid to form a carboxylate and then reacting it with a primary alkyl halide using an SN2 reaction.
Reactions of Esters
- Esters are one of the more useful functional groups.
- Their low reactivity makes the easy to work with and they are stable enough to be used as a solvent in organic reactions (ex. ethyl acetate).
- Esters are still reactive enough to undergo hydrolysis to form carboxylic acids, alcoholysis, to form different esters, and aminolysis to form amides.
- they can react with Grignard reagents to form 3o alcohols and hydride reagents to form 1o alcohols or aldehydes.
Conversion of Esters to Carboxylic Acids
Hydrolysis
- Esters can be converted back into a carboxylic acid and an alcohol through hydrolysis with water and a catalytic amount of strong acid.
- This reaction represents the reverse of the acid catalyzed esterification of a carboxylic acid and an alcohol.
- Both the ester formation and cleavage reactions are part of an equilibrium which can be manipulated using Le Chatelier’s principle.
- For ester hydrolysis, the equilibrium is shifted toward carboxylic acid formation by using a large excess of water in the reaction.
General Reaction
Example
Mechanism
- Acid catalysis is required during ester hydrolysis due to water being a weak nucleophile.
- Protonation of the ester carbonyl increases the partial positive charge on the carbonyl carbon increasing its electrophilicity.
- water adds to the carbonyl carbon causing the formation of a tetrahedral alkoxide intermediate.
- A proton transfers to the –OR group, increasing its ability to act as a leaving group.
- Reforming the carbonyl double bond causes the elimination of an alcohol (HOR) as a leaving group, creating a protonated carboxylic acid.
- lastly, water acts as a base, removing a hydrogen, to form a carboxylic acid and regenerating the acid catalyst.
1) Protonation of the carbonyl
2) Nucleophilic attack by water
3) Proton transfer
4) Leaving group removal
5) Deprotonation
Hydrolysis of Cyclic esters
e.g lactones undergo typical reactions of esters including hydrolysis. Hydrolysis of the lactone under acidic conditions creates a hydroxy acid.
Conversion of Esters to Carboxylic Acids
Saponification
- Esters can also be cleaved into a carboxylate and an alcohol through reaction with water and a base.
- Saponification reaction utilize a better nucleophile (hydroxide) and are typically faster than an acid catalyzed hydrolysis.
- The carboxylation ions produced by saponification are negatively charged and very unreactive toward further nucleophilic substitution which makes the reaction irreversible.
General Reaction
Example
Mechanism
- the nucleophilic addition of a hydroxide ion at the carbonyl carbon to give a tetrahedral alkoxide intermediate.
- The carbonyl bond is reformed along with the elimination of an alkoxide (-OR) leaving group yielding a carboxylic acid.
- The alkoxide base deprotonates the carboxylic acid to for a carboxylate salt and an alcohol as products.
- deprotonation step essentially removes the carboxylic acid from the equilibrium which drives the saponification towards completion.
1) Nucleophilic attack by hydroxide
2) Leaving group removal
3) Deprotonation
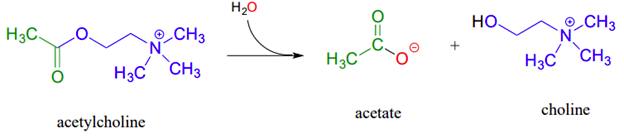
Ester saponification in biological systems
Example, acetylcholinesterase, an enzyme present in the synapse, catalyzes hydrolysis of the ester group in acetylcholine which is a neurotransmitter that triggers muscle contraction. The acetylcholinesterase reaction proceeds in two phases:
- a covalent enzyme-substrate intermediate is formed when the acyl group of acetylcholine is transferred to an active-site serine on the enzyme (a transesterification reaction).
- A water nucleophile then attacks this ester, driving off acetate and completing the hydrolysis
Conversion of Ester to Amides
It is possible to convert esters to amides through direct reaction with ammonia or amines. However, these reactions are not commonly used because the formation of an amide using an acid chloride is a much simpler reaction.
Conversion of Esters to 1o Alcohols
Hydride Reduction
- Esters can undergo hydride reduction with LiAlH4 to form two alcohols.
- The alcohol derived from the acyl group of the ester will be 1o and is typically considered the main product of the reaction.
- The other alcohol is derived from the ester’s alkoxy group and is typically considered a side-product of the reaction.
- Sodium borohydride (NaBH4) is not a reactive enough hydride agent to reduce esters or carboxylic acids.
- NaBH4 can selectively reduce aldehydes and ketones in the presence of ester functional groups.
General Reaction
Predicting the Products of a Hydride Reduction
during this reaction, there are three major changes in bonding:
1) The –OR leaving group is removed from the ester.
2) The C=O carbonyl bond is converted to a C-O-H, an alcohol.
3) Two C-H bonds are formed as two of the hydride nucleophiles are added to the original carbonyl carbon of the ester.
Example
Mechanism
- Nucleophilic acyl substitution replaces the –OR leaving group in ester with a hydride nucleophile to form an aldehyde intermediate.
- Because aldehydes are more reactive than esters, they rapidly undergo a second nucleophilic hydride addition to form a tetrahedral alkoxide intermediate.
- An acid work-up protonates the alkoxide to create a 1o alcohol.
1) Nucleophilic attack by the hydride
2) Leaving Group Removal
3) Nucleopilic attack by the hydride anion
4) The alkoxide is protonated
Conversion of Esters to Aldehydes
Hydride Reduction
- esters can be converted to aldehydes using the weaker reducing reagent diisobutylaluminum hydride (DIBALH).
- The reaction is usually carried out at -78 oC to help isolate the aldehyde product.
General Reaction
Example
Conversion of Esters to 3o Alcohols
Grignard Reagents
- Addition of Grignard reagents converts esters to two alcohols, one 3o alcohols (main product) and one 1o alcohol (considered a side product).
- The Grignard reagent adds to the ester twice, once during a nucleophilic acyl substitution to form a ketone intermediate then again during a nucleophilic addition to form the 3o alcohol product.
- Overall, during this reaction two C-C bonds are formed on the ester’s original carbonyl carbon.
General Reaction
Predicting the Products of a Grignard Reaction
Example
Mechanism
1) Nucleophilic Attack
2) Leaving group removal
3) Nucleophilic attack
4) Protonation