Electrolysis
- It is the breakdown of an electrolyte- ionic compound, molten or aqueous solution- by passing an electric current
- This is possible due to the presence of mobile electrons
Electrolyte
- Aqueous solution of ionic substance or molten ionic salt
- Conducts electricity due to the presence of mobile ions
| Components of Electrolysis | Definition |
| Electrodes | Metal or graphite rods that aid the flow of electricity in and out of the electrolyte 1. Anode: Positive electrode 2. Cathode: Negative ElectrodeReactive electrodes take part in the reaction while inert electrodes do not |
| Anion | Negatively charged ion that moves to anode |
| Cation | Positively charged ion that moves to cathode |

Principle
- Reduction of positive cations happens at the cathode
- Oxidation of negative anions happens at the anode
For example:
- At the anode: 2Cl– → Cl2 + 2e–
- At the cathode: 2H+ + 2e– → H2
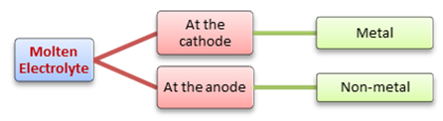

Examples of Electrolysis
| Electrolyte | At cathode | At anode |
| Molten lead (II) bromide | Lead | Bromine |
| Concentrated hydrochloric acid | Hydrogen | Chlorine |
| Concentrated aqueous sodium chloride | Hydrogen | Chlorine |
| Dilute sulfuric acid | Hydrogen | Oxygen |
Voltaic Cell
- Used to produce electrical energy from chemical energy
- The electrodes are made from metals with different reactivity
1.Negative electrode: More reactive metal, ex. Zinc
- Positive electrode: Less reactive metal, ex. Iron
- Electrolyte is a strong acid, ex. sulfuric acid
- The negative electrode loses electrons; these flow through the simple circuit to the positive electrode to produce voltage. This is measured on the attached voltmeter.
Note: The greater the difference in reactivity, the greater voltage produced
Electroplating
The process of coating the surface of a metal (more reactive) with another metal (less reactive) using electrolysis
-
Components:
- Anode: pure metal being used to electroplate the object
- Cathode: object being electroplated
- Electrolyte: aqueous solution of the soluble salt of pure metal (same as anode)
-
Used to:
- Prevent corrosion
- Enhance appearance
| Conductors | Insulators |
| Allow the passage of electrical charge | Resist the passage of electrical charge |
| Aluminum [low density, non-corrosive, cheaper than copper]: used in electricity cables with a steel core and plane bodies | Plastics for casing in wires |
| Copper [malleable]: used in electrical wires | Ceramics used to support cables in electricity pylons |
Electrolytic purification of metals
- Cathode: thin strip of pure metal
- Anode: impure metal
- Electrolyte: Aqueous Salt Solution of metal
Purification of copper
Impure copper as the anode and pure copper as the cathode; the aqueous copper (II) sulfate helps the copper ions move from the anode to the cathode. Here the ions gain electrons and become copper atoms, making the pure copper cathode thicker.
- Reaction at Anode: Cu – 2e Cu2+ (mass decreases)
- Reaction at Cathode: Cu2+ + 2e Cu (mass increases)

Electrolytic purification of copper
- Electrolyte: aq copper(ii)sulphate
- Cathode: thin strip of pure copper
- Anode: impure metal
- Copper ions from the solution lose thier charge and copper is deposited at the cathode, mass increases
- Copper atoms lose their valency electrons and go into the solution as ions at the anode, mass decreases
Electroplating
Electroplating is done to coat a metal with another metal to improve its appearance or to improve its resistance to corrosion. The metal used for plating is used as the anode and the object to be electroplated acts as the cathode. The electrolyte used is the salt solution of the metal used for
plating.

Copper metal acts as the anode as it is used to plate the object. The electrolyte used is a salt solution of its salt (copper(II) sulfate solution) and the object to be plated acts as the cathode.
At the anode, the copper atoms are oxidised into Cu2+ ions, which enter the electrolyte. At the cathode, Cu2+ ions are discharged and deposited on the object, plating it with
copper metal.
Extraction of Aluminum
- The main ore of aluminum is bauxite – high m.p.
- Aluminum (III) oxide (alumina) is dissolved in molten cryolite (Na3AlF6) – this mixture has a lower m.p. (industrially preferred)

- During electrolysis aluminum ( Al3+ + 3e- → Al ) is produced at the carbon cathode and oxygen ( 2O2- – 4e- → O2 ) at the carbon anode.
- Due to the high temp. the oxygen reacts with the carbon in the graphite anode to form CO2 and so anode has to be periodically replaced
Electrolysis of Brine
- Brine is concentrated aqueous NaCl solution
- Ions present: Na+, H+, Cl– and OH–

| At the anode | At the cathode |
| Made of titanium | Made of steel |
| Cl– ions; Chlorine gas | Hydrogen cations reduced to H2 molecules |
| Unreacted ions (Na+, H+ and OH–) move through porous membrane due to difference in liquid pressure |
| Left Na+ and OH– which form aqueous sodium hydroxide |
73 Comments
tlovertonet
May 15, 2024I’m so happy to read this. This is the kind of manual that needs to be given and not the accidental misinformation that is at the other blogs. Appreciate your sharing this greatest doc.
tlovertonet
May 23, 2024Spot on with this write-up, I actually think this website needs far more consideration. I’ll probably be again to learn rather more, thanks for that info.
Glucotrust Reviews
June 15, 2024Glad to be one of several visitants on this awe inspiring website : D.
SightCare review
June 16, 2024I’m really enjoying the theme/design of your web site. Do you ever run into any web browser compatibility problems? A number of my blog audience have complained about my site not working correctly in Explorer but looks great in Chrome. Do you have any solutions to help fix this problem?
Ikaria Lean Belly Juice reviews
June 18, 2024Hello, Neat post. There’s an issue together with your website in internet explorer, could test this?K IE nonetheless is the marketplace chief and a huge part of other folks will leave out your fantastic writing due to this problem.
Sugar Defender
June 22, 2024Great write-up, I am regular visitor of one?¦s blog, maintain up the nice operate, and It is going to be a regular visitor for a lengthy time.
Prodentim
June 23, 2024Utterly composed subject matter, Really enjoyed examining.
water damage mitigation roswell
June 25, 2024Some truly quality posts on this website , saved to fav.
SeroLean Review
June 25, 2024It’s a pity you don’t have a donate button! I’d certainly donate to this fantastic blog! I suppose for now i’ll settle for book-marking and adding your RSS feed to my Google account. I look forward to brand new updates and will talk about this website with my Facebook group. Talk soon!
hire a hacker for iPhone
June 26, 2024Thanks a bunch for sharing this with all of us you actually know what you are talking about! Bookmarked. Please also visit my site =). We could have a link exchange contract between us!
cognicare pro
June 30, 2024What is CogniCare Pro? CogniCare Pro is 100 natural and safe to take a cognitive support supplement that helps boost your memory power. This supplement works greatly for anyone of any age and without side effects
free online webinars
July 3, 2024Its like you read my mind! You seem to know so much about this, like you wrote the book in it or something. I think that you can do with a few pics to drive the message home a little bit, but instead of that, this is wonderful blog. A great read. I will certainly be back.
santé
July 3, 2024I went over this internet site and I think you have a lot of wonderful information, saved to fav (:.
alpaca wool blanket queen size
July 3, 2024I’m very happy to read this. This is the kind of manual that needs to be given and not the random misinformation that’s at the other blogs. Appreciate your sharing this greatest doc.
derfor sa-co produkter til auto branchen og industri
July 3, 2024You are my intake, I possess few blogs and occasionally run out from to brand : (.
Zencortex
July 5, 2024You need to take part in a contest for top-of-the-line blogs on the web. I will suggest this site!
zoritoler imol
July 5, 2024Great V I should certainly pronounce, impressed with your site. I had no trouble navigating through all the tabs and related information ended up being truly simple to do to access. I recently found what I hoped for before you know it at all. Quite unusual. Is likely to appreciate it for those who add forums or something, site theme . a tones way for your client to communicate. Nice task..
tlover tonet
July 7, 2024I think other website proprietors should take this web site as an model, very clean and fantastic user friendly style and design, as well as the content. You are an expert in this topic!
Fitspresso
July 7, 2024It is in reality a great and helpful piece of information. I am happy that you just shared this helpful information with us. Please stay us informed like this. Thank you for sharing.
hire a hacker for instagram
July 10, 2024It’s really a great and helpful piece of info. I am glad that you shared this useful information with us. Please keep us up to date like this. Thank you for sharing.
Cogni Care pro review
July 13, 2024Greetings from California! I’m bored to tears at work so I decided to check out your blog on my iphone during lunch break. I really like the info you present here and can’t wait to take a look when I get home. I’m shocked at how fast your blog loaded on my phone .. I’m not even using WIFI, just 3G .. Anyways, excellent site!
Uyap Uyumlu Call Center Yazılımı
July 14, 2024I like this post, enjoyed this one appreciate it for putting up. “The reward for conformity was that everyone liked you except yourself.” by Rita Mae Brown.
hit lồn mẹ vợ
July 14, 2024I like what you guys are up also. Such clever work and reporting! Keep up the excellent works guys I have incorporated you guys to my blogroll. I think it will improve the value of my web site 🙂
garansi kekalahan
July 15, 2024Thanks a lot for sharing this with all of us you really know what you’re talking about! Bookmarked. Please also visit my site =). We could have a link exchange agreement between us!
milanslot
July 15, 2024This really answered my problem, thank you!
agencija za digitalni marketing
July 15, 2024hello!,I like your writing so so much! proportion we communicate extra about your article on AOL? I require an expert on this space to unravel my problem. Maybe that’s you! Looking ahead to see you.
muliaplay
July 15, 2024Hi! I know this is kinda off topic nevertheless I’d figured I’d ask. Would you be interested in exchanging links or maybe guest authoring a blog article or vice-versa? My site addresses a lot of the same topics as yours and I believe we could greatly benefit from each other. If you are interested feel free to shoot me an email. I look forward to hearing from you! Awesome blog by the way!
ice rock
July 16, 2024I’ve recently started a website, the info you provide on this site has helped me tremendously. Thanks for all of your time & work. “The man who fights for his fellow-man is a better man than the one who fights for himself.” by Clarence Darrow.
ikaria lean belly juice
July 16, 2024You could definitely see your expertise in the work you write. The world hopes for more passionate writers like you who are not afraid to say how they believe. Always go after your heart.
parede acustica Drywall em Guarulhos
July 17, 2024My brother recommended I might like this website. He was entirely right. This post actually made my day. You can not imagine simply how much time I had spent for this information! Thanks!
mercedes service near me
July 17, 2024I’ll immediately seize your rss feed as I can’t to find your e-mail subscription link or newsletter service. Do you’ve any? Kindly let me recognise so that I could subscribe. Thanks.
obor138
July 17, 2024I do enjoy the way you have framed this specific matter plus it does offer us a lot of fodder for consideration. On the other hand, coming from just what I have personally seen, I simply just wish when the responses stack on that men and women continue to be on issue and not embark on a soap box of the news of the day. Still, thank you for this exceptional piece and whilst I can not necessarily concur with it in totality, I respect the viewpoint.
instagram hackers for hire
July 17, 2024Thank you for the auspicious writeup. It in fact was a amusement account it. Look advanced to far added agreeable from you! However, how can we communicate?
causas de zumbidos nos ouvidos
July 18, 2024Great write-up, I?¦m normal visitor of one?¦s site, maintain up the excellent operate, and It’s going to be a regular visitor for a long time.
Casa em Cascatinha
July 18, 2024Hello, i think that i saw you visited my blog thus i came to “return the favor”.I am attempting to find things to enhance my web site!I suppose its ok to use some of your ideas!!
jual tenda membrane
July 18, 2024Some genuinely nice and useful info on this web site, as well I conceive the design and style contains great features.
krikya online
July 19, 2024Thank you, I have just been searching for info about this subject for ages and yours is the best I have discovered till now. But, what about the conclusion? Are you sure about the source?
krikya online
July 19, 2024I love it when people come together and share opinions, great blog, keep it up.
krikya login
July 19, 2024Hello! I could have sworn I’ve been to this website before but after checking through some of the post I realized it’s new to me. Anyhow, I’m definitely delighted I found it and I’ll be bookmarking and checking back frequently!
wat te doen tegen inhammen
July 20, 2024I’m curious to find out what blog system you have been using? I’m experiencing some minor security problems with my latest website and I would like to find something more safeguarded. Do you have any suggestions?
https://pa-sukabumi.go.id/draft/
July 20, 2024Great wordpress blog here.. It’s hard to find quality writing like yours these days. I really appreciate people like you! take care
porn talk ai
July 21, 2024I have been absent for some time, but now I remember why I used to love this blog. Thank you, I’ll try and check back more often. How frequently you update your site?
Cellucare
July 21, 2024This is a very good tips especially to those new to blogosphere, brief and accurate information… Thanks for sharing this one. A must read article.
koperasi1malaysia
July 22, 2024Youre so cool! I dont suppose Ive read anything like this before. So good to seek out somebody with some original thoughts on this subject. realy thank you for starting this up. this web site is something that is needed on the internet, someone with a bit of originality. useful job for bringing something new to the internet!
TBS Car Battery
July 22, 2024What i do not realize is in fact how you are no longer actually a lot more smartly-preferred than you might be right now. You are so intelligent. You already know thus considerably when it comes to this matter, made me individually believe it from so many various angles. Its like women and men are not interested unless it’s one thing to accomplish with Girl gaga! Your personal stuffs excellent. Always care for it up!
OneWave Home Solar Water Heater, Boiler, Water Pump & Water Filter
July 22, 2024Thank you for sharing superb informations. Your website is very cool. I’m impressed by the details that you have on this site. It reveals how nicely you perceive this subject. Bookmarked this website page, will come back for more articles. You, my pal, ROCK! I found simply the information I already searched everywhere and simply could not come across. What an ideal web-site.
https://techtraacademy.my/ch
July 22, 2024Its wonderful as your other posts : D, regards for putting up.
cabin crew diploma
July 22, 2024You could certainly see your skills in the paintings you write. The sector hopes for more passionate writers such as you who are not afraid to say how they believe. At all times follow your heart.
agen properti
July 23, 2024Perfectly indited written content, Really enjoyed looking at.
raja76
July 23, 2024I do enjoy the manner in which you have presented this specific matter plus it does indeed provide me a lot of fodder for consideration. However, coming from just what I have experienced, I basically wish as the remarks pack on that individuals continue to be on point and in no way embark on a tirade regarding the news of the day. All the same, thank you for this exceptional piece and while I can not really agree with it in totality, I value the standpoint.
Audionex analise
July 23, 2024Can I just say what a relief to find someone who actually knows what theyre talking about on the internet. You definitely know how to bring an issue to light and make it important. More people need to read this and understand this side of the story. I cant believe youre not more popular because you definitely have the gift.
sell any car
July 23, 2024Amazing! This blog looks exactly like my old one! It’s on a entirely different topic but it has pretty much the same page layout and design. Outstanding choice of colors!
Java Burn
July 23, 2024I discovered your blog site on google and check a few of your early posts. Continue to keep up the very good operate. I just additional up your RSS feed to my MSN News Reader. Seeking forward to reading more from you later on!…
aplikasi freelancer
July 23, 2024WONDERFUL Post.thanks for share..extra wait .. …
rent a car otopeni
July 24, 2024Really enjoyed this post, how can I make is so that I receive an email when you publish a new post?
hire a hacker for snapchat
July 24, 2024I would like to thank you for the efforts you’ve put in writing this blog. I’m hoping the same high-grade website post from you in the upcoming as well. Actually your creative writing skills has inspired me to get my own web site now. Actually the blogging is spreading its wings rapidly. Your write up is a great example of it.
Sight Care reviews
July 24, 2024I like what you guys are up too. Such smart work and reporting! Keep up the superb works guys I have incorporated you guys to my blogroll. I think it’ll improve the value of my website 🙂
driveway pavers
July 24, 2024Aw, this was a really nice post. In idea I wish to put in writing like this additionally – taking time and actual effort to make a very good article… however what can I say… I procrastinate alot and under no circumstances seem to get one thing done.
etnico busto arsizio
July 24, 2024magnificent points altogether, you just gained a brand new reader. What might you suggest in regards to your put up that you made some days in the past? Any sure?
commercial duct cleaning near me
July 24, 2024Nice post. I learn something more challenging on different blogs everyday. It will always be stimulating to read content from other writers and practice a little something from their store. I’d prefer to use some with the content on my blog whether you don’t mind. Natually I’ll give you a link on your web blog. Thanks for sharing.
concursos em sc
July 24, 2024I like what you guys are up too. Such intelligent work and reporting! Carry on the superb works guys I have incorporated you guys to my blogroll. I think it will improve the value of my website 🙂
read the full info here
July 24, 2024Please let me know if you’re looking for a author for your weblog. You have some really good posts and I feel I would be a good asset. If you ever want to take some of the load off, I’d really like to write some articles for your blog in exchange for a link back to mine. Please send me an e-mail if interested. Kudos!
Premium Explainer Video Production Agency
July 24, 2024This blog is definitely rather handy since I’m at the moment creating an internet floral website – although I am only starting out therefore it’s really fairly small, nothing like this site. Can link to a few of the posts here as they are quite. Thanks much. Zoey Olsen
suzuki bandung
July 25, 2024Awsome info and right to the point. I don’t know if this is actually the best place to ask but do you people have any thoughts on where to get some professional writers? Thanks 🙂
Imóveis para venda Santa cruz do sul
July 25, 2024I was very pleased to search out this internet-site.I wanted to thanks in your time for this excellent read!! I undoubtedly having fun with every little little bit of it and I’ve you bookmarked to check out new stuff you blog post.
ceria138
July 25, 2024I just could not depart your site prior to suggesting that I actually loved the standard information an individual provide for your visitors? Is gonna be back ceaselessly in order to investigate cross-check new posts.
wedding photographers south florida
July 25, 2024Very interesting topic, appreciate it for posting.
miami artist
July 25, 2024I really like your writing style, superb information, thank you for posting :D. “Kennedy cooked the soup that Johnson had to eat.” by Konrad Adenauer.
Como conseguir um comprovante de residência
July 25, 2024I have been browsing on-line more than 3 hours nowadays, yet I never found any fascinating article like yours. It is lovely worth enough for me. In my opinion, if all website owners and bloggers made just right content material as you did, the internet will probably be much more useful than ever before. “No one has the right to destroy another person’s belief by demanding empirical evidence.” by Ann Landers.
like this
July 25, 2024A person essentially lend a hand to make significantly articles I might state. That is the first time I frequented your website page and up to now? I surprised with the research you made to create this particular put up incredible. Excellent activity!
Caminão Limpa Fossa no Lago Sul DF
July 25, 2024Thank you for the auspicious writeup. It in reality was a amusement account it. Look complex to far brought agreeable from you! However, how can we be in contact?
ninjagacor
July 26, 2024Heya i am for the first time here. I found this board and I find It really useful & it helped me out much. I hope to give something back and aid others like you helped me.
Kesehatan
July 26, 2024Great amazing issues here. I?¦m very satisfied to peer your article. Thanks a lot and i am taking a look ahead to touch you. Will you please drop me a e-mail?