Characteristic physical properties
Group 17 – the halogens – consists of reactive non-metals, all of which exist as diatomic molecules, X2. Their properties are summarised in below
| Element | Appearance | Boiling point/K | E⦵ for X2 + 2e− ⇌ 2X−/V |
Electronegativity |
| Fluorine | Pale-yellow gas | 085 | +2.87 | 4.0 |
| Chlorine | Yellow-green gas | 238 | +1.36 | 3.0 |
| Bromine | Dark-red liquid | 332 | +1.07 | 2.8 |
| Iodine | Shiny dark-grey solid | 457 | +0.54 | 2.5 |
- The Group 17 elements are volatile non-metals. They exist as diatomic molecules that attract each other using van der Waals’ forces (these are sometimes referred to as instantaneous dipole–induced dipole forces).
- The larger the halogen molecules, the bigger the van der Waals’ forces, and hence the higher the boiling point.
- There is a decrease in reactivity on moving down the group. This is due, in part, to the increase in atomic radius because the incoming electron has to go into a shell further away from an increasingly shielded nucleus.
- Nonetheless, the elements are still too reactive to occur uncombined, unlike some metals and other non-metals such as carbon.
- The electronegativity and the E⦵ values show that these elements are oxidising agents with reactivity that decreases down the group.
Important chemical reactions of hydrogen halides
One of the most important set of compounds of the Group 17 elements is the hydrogen halides, HX. These are prepared in different ways depending on the oxidising power of the halogen concerned. Hydrogen chloride can be prepared by heating sodium chloride with concentrated sulfuric acid:

However, neither hydrogen bromide nor hydrogen iodide can be prepared by this method because they would be oxidised further by the sulfuric acid.
The only acid that can be used to prepare all three hydrogen halides is phosphoric(V) acid:

Hydrogen fluoride is much harder to produce in a pure state because fluorine is such a strong oxidising agent. It will even oxidise water, giving a mixture of hydrogen fluoride and oxygen:

You have to be able to compare the bond energies of the hydrogen halides and use these data to explain their relative thermal stabilities.
| Element | Bond energy/kJ mol−1 | |||
| Fluoride | Chloride | Bromide | Iodide | |
| H | 568 | 432 | 366 | 298 |
| C | 467 | 346 | 290 | 228 |
The bond energies of fluorine with hydrogen and carbon are significantly higher than those of the other Group 17 elements. This means that the formation of covalent fluorides is usually strongly exothermic because this means breaking F–F bonds and making E–F bonds (where E is the element concerned).
H–X bond energies (where X is the halogen) decrease steadily down the group, making it easier to break the H–X bond. So, for example, plunging a red-hot wire into a halogen halide has the following effects.
-
.
Reactions of the halide ions, other than fluoride ions
Testing for halide ions
The characteristic test for halide ions in solution is to add silver nitrate solution followed by aqueous ammonia. You have probably carried out this test a number of times in practical sessions.
| Halide ion | Reaction with Ag+(aq) | Subsequent reaction with NH3(aq) |
| Chloride | White precipitate is formed | Dissolves to form colourless solution |
| Bromide | Cream precipitate is formed | Only dissolves in concentrated ammonia |
| Iodide | Yellow precipitate is formed | Insoluble in ammonia |

The equilibrium in the first equation lies to the right so it can be regarded as a forward reaction. However, the equilibrium in the second equation also lies to the right and, therefore, removes silver ions from the remaining solution, causing the silver halide to dissolve. This is true for chloride and bromide ions. Silver iodide is so insoluble that even concentrated ammonia solution is unable to reverse the process.
Reactions with other halogens
The halide ions in aqueous solution react as reducing agents with halogens higher up the group, being oxidised to their respective halogen. Aqueous chlorine reacts with both bromide ions and iodide ions, liberating bromine and iodine respectively:
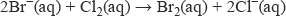
Aqueous bromine liberates iodine from iodide ions.
Reactions with concentrated sulfuric acid
The reactions of the halide ions with concentrated sulfuric acid can also be used as a test.
| Ion present | Observations |
| Chloride | Steamy acidic fumes (of HCl) |
| Bromide | Steamy acidic fumes (of HBr) mixed with brown bromine vapour |
| Iodide | Some steamy fumes (of HI) and lots of purple iodine vapour |
Although a hydrogen halide is formed in each case, hydrogen bromide and hydrogen iodide are oxidised by the sulfuric acid:

Note that one molecule of sulfuric acid oxidises two molecules of hydrogen bromide, but eight molecules of hydrogen iodide. This shows how much easier it is to oxidise iodide ions, I−, than it is to oxidise bromide ions, Br−.
dissolve. This is true for chloride and bromide ions. Silver iodide is so insoluble that even concentrated ammonia solution is unable to reverse the process.
Reactions with other halogens
The halide ions in aqueous solution react as reducing agents with halogens higher up the group, being oxidised to their respective halogen. Aqueous chlorine reacts with both bromide ions and iodide ions, liberating bromine and iodine respectively:
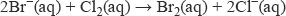
Aqueous bromine liberates iodine from iodide ions.
Reactions with concentrated sulfuric acid
The reactions of the halide ions with concentrated sulfuric acid can also be used as a test.
| Ion present | Observations |
| Chloride | Steamy acidic fumes (of HCl) |
| Bromide | Steamy acidic fumes (of HBr) mixed with brown bromine vapour |
| Iodide | Some steamy fumes (of HI) and lots of purple iodine vapour |
Although a hydrogen halide is formed in each case, hydrogen bromide and hydrogen iodide are oxidised by the sulfuric acid:

Note that one molecule of sulfuric acid oxidises two molecules of hydrogen bromide, but eight molecules of hydrogen iodide. This shows how much easier it is to oxidise iodide ions, I−, than it is to oxidise bromide ions, Br−.
Reactions of chlorine with sodium hydroxide
Chlorine reacts differently with sodium hydroxide depending on the temperature and the concentration of the alkali.
With cold, dilute sodium hydroxide solution the reaction is:
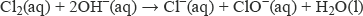
In this reaction, the element chlorine (oxidation number 0) has been converted into chloride ions (oxidation number −1) and chlorate(I) ions (oxidation number +1).
With hot, concentrated sodium hydroxide solution this reaction takes place:
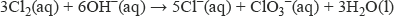
In this case, the element chlorine has been converted into chloride ions (oxidation number −1) and chlorate(V) ions (oxidation number +5).
These are both examples of disproportionation reactions in which an element is both oxidised and reduced.
The reason for the difference in the two reactions is the instability of the chlorate(I) ion, in which the chlorine disproportionates at higher temperatures:

Important use of halogens and halogen compounds
Chlorine and, to a lesser extent, the other halogens have a number of important economic and industrial uses.
Chlorine in water purification
- Chlorine is used to kill bacteria and sterilise water for domestic supplies in many parts of the world – it is also used in some swimming pools.
- The ability of chlorine to destroy bacteria is a result of its powerful oxidising power, which disrupts the chemistry of bacterial cells.
- However, traces in our drinking water are insufficient to do us any harm and the benefits of water chlorination far outweigh the risks. The chlorine may be supplied as the gas or added as solid sodium chlorate(I).
Manufacture of bleach
Sodium hydroxide and chlorine can be combined chemically to make the bleach, sodium chlorate(I), NaClO. This is used in some domestic cleaning agents. It chemically cleans materials such as washbasins and toilets, and ‘kills’ microorganisms.
Practice questions
- describe (and explain where appropriate) the physical properties of the Group 17 elements chlorine, bromine and iodine – colour, trend in volatility and bond strength
- describe the relative reactivity of the Group 17 elements as oxidising agents
- describe the reactions of the Group 17 elements with hydrogen and explain the relative reactivity in these reactions; describe the relative thermal stabilities of the hydrogen halides and explain these in terms of bond length
- describe the relative reactivity of halide ions as reducing agents
- describe and explain the reactions of halide ions with aqueous silver ions followed by ammonia; the reactions with concentrated sulfuric acid with balanced equations
- describe and explain the reactions of chlorine with hot and cold aqueous sodium hydroxide and the use of chlorine in water purification.