Acids
- An acid is a compound which when dissolved in water produces hydrogen ions (H+ / proton)
- acids in daily life includes ethanoic acid found in vinegar & tomato juice, citric acids found in citrus foods, lactic acid found in sour milk & in muscle respiration….
- common lab acids include hydrchloric acid, sulphuric acid and nitric acid
- Strong acids completely ionize in water producing lots of H+ ions
- Weak acids partially ionize in water producing few H+ ions
Properties of Acids
An acid is a substance that produces hydrogen ions (H+) when dissolved in water. Acids are proton donors.
Indicators
- Have pH between 1 (strong) and 6 (weak)
- Turns blue litmus red
- Turns methyl orange indicator red
Strong acids completely ionize in water producing lots of H+ ions
Weak acids partially ionize in water producing few H+ ions
Chemical properties
- Acid + metal → salt + hydrogen gas
- Acid + base → salt + water
- Acid + metal carbonate → salt + carbon dioxide + water
Properties of Bases
Bases are substances which neutralize acids to form a salt and water only. They are proton acceptors (form OH– ions) and are mainly water insoluble.
- Have pH 8 to 14.
- alkalis have a slippery feel
- Alkalis are hazardous, dilute alkalis are irritants & concentrated alkalis are corrosive and brn skin
- Alkalis turn red litmus indicator paper (or solution) to blue.
- Base + acid → salt + water (+ CO2 when base is a metal carbonate)
- Base + ammonium salt → salt + ammonia gas + water
- Strong alkalis completely ionize in water producing lots of OH- ions
- Weak alkalis partially ionize in water producing OH- ions
Indicators
- Have pH between 8 (weak) and 14 (strong)
- Turns red litmus blue
- Turns methyl orange indicator yellow
Strong alkalis completely ionize in water producing lots of OH– ions
Weak alkalis partially ionize in water producing OH– ions
Neutral
- Neutral substances are pH 7.
- Acidity in soil:
Optimal plant growth requires a soil pH between 5 and 8. Soil pH imbalance to be too acidic reduces plant growth yield. Soil acidity is neutralized by adding lime or powdered limestone.
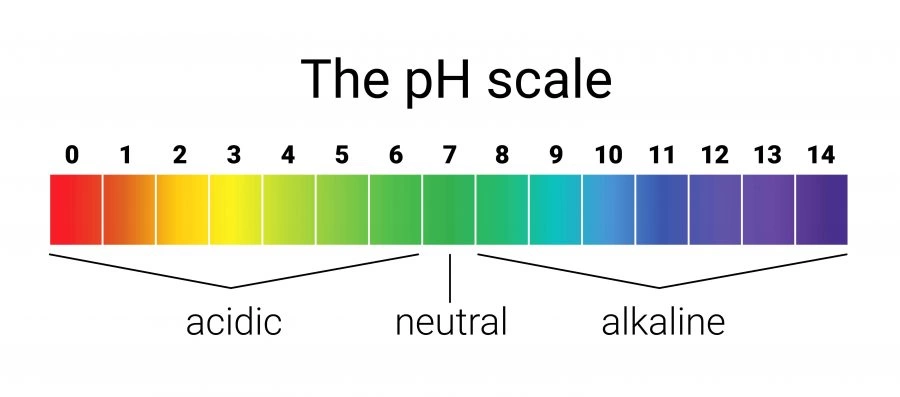
pH scale
pH is the concentration of H+ ions per dm3 of solution
Universal indicator solution is used to determine the pH of a substance by matching the color change to the pH color chart.
| Indicator | Colour in acids | Color in alkalis |
|---|---|---|
| Phenolphalein | colourless | yellow |
| Methyl orange | red | yellow |
| bromothylmol blue | yellow | blue |
| litmus | red | blue |
Types of Oxides
- Metal oxides are basic in nature e.g. iron oxide and magnesium oxide
- Non-metal oxides are acidic in nature e.g. sulphur oxide and carbon dioxide
- Aluminum, zinc and lead form amphoteric oxides e.g. zinc oxide
- Oxides that react with neither acids nor bases are neutral e.g. nitric oxide and carbon monoxide
Preparation of Salts
- A salt is a compound formed when all the hydrogen atoms of an acid are replaced by a metal.
- Naming salts involves 2 parts; the name of the metal and the acid ending
-
calcium + hydrochloric acid = calcium chloride
| Type of Salt | Acid used |
| Sulphate | Sulphuric acid |
| Nitrate | Nitric acid |
| Chloride | Hydrochloric acid |
| Ethanoate | Ethanoic acid |
Salts can either be soluble or insoluble
| Soluble Salts | Insoluble Salts |
| All sodium, potassium and ammonium salts | |
| All nitrates | |
| Chlorides | Except silver and lead |
| Sulphates | Except barium, lead and calcium |
| Potassium, sodium and ammonium carbonates | All other carbonates |
Preparation of Soluble Salts
Method A: Neutralization
- Put a certain amount alkali in a flask
- Add phenolphthalein
- Add acid from a burette, stirring, until it goes colorless
- Find out how much acid you used
- Repeat, to be more accurate
- Evaporate water from neutral solution
Method B: Titration
- Phenolphthalein is added to an alkali (soluble base)
- Add acid to solution using burette; note volume of acid required for solution to change color
- Repeat without indictor using noted acid volume
- Heat in evaporating dish to form soluble salt crystals
Preparation of Insoluble Salts
Method C: Precipitation
- 2 soluble salts added to water and mixed Note: one soluble salt should always be a potassium or sodium solution (eg. potassium sulfate)
- Filter out and clean precipitate with distilled water
- Dry insoluble salt precipitate in oven
Test for Aqueous Cations
| Cation | with aqueous NaOH | with aqueous Ammonia |
| Aluminum (Al3+) | White soluble precipitate, turns colorless in excess | White precipitate, insoluble in excess |
| Ammonium (NH4+) | Pungent ammonium gas produced turns damp red litmus blue | |
| Calcium (Ca2+) | White precipitate, insoluble in excess | Faint or no precipitate |
| Copper (Cu2+) | Blue precipitate, insoluble in excess | Blue precipitate, soluble in excess to give a dark blue solution |
| Iron(II) (Fe2+) | Dirty green precipitate, insoluble in excess | Dirty green precipitate, soluble in excess |
| Iron(III) (Fe3+) | Reddish-brown precipitate, insoluble in excess | Reddish-brown precipitate, insoluble in excess |
| Zinc (Zn2+) | White precipitate, soluble and turns colorless in excess | White precipitate, soluble and turns colorless in excess |
| Chromium (Cr3+) | Grey green precipitate, soluble to give dark green solution in excess | Grey green precipitate, insoluble in excess |
Test for Anions
Sulfate ions (SO42-):
Add dilute nitric acid, then add aq. barium nitrate. White precipitate formed\
Sulphite ions (SO32-):
Add acidified potassium permanganate and heat. Color changes from pink to colorless
Halide ions:
Add nitric acid, then aqueous silver nitrate
| Chloride (Cl–) | White precipitate |
| Bromide (Br–) | Cream precipitate |
| Iodide (I–) | Yellow precipitate |
Nitrate ions (NO3–):
Add aqueous sodium hydroxide then add warm aluminum foil. Pungent gas produced, turns damp red litmus blue
Carbonate ions (CO32-):
Add dilute hydrochloric acid. If bubbles/ gas produced turn limewater cloudy, carbonate ion present
Test for Gases
| Gas | Test and test result |
| Ammonia (NH3) | Damp red litmus paper turns blue |
| Carbon dioxide (CO2) | Bubble gas through–from colorless to cloudy |
| Chlorine (Cl2) | Bleaches red/blue litmus paper |
| Hydrogen (H2) | Place lighted splint, squeaky pop |
| Oxygen (O2) | Place glowing splint, splint relights |
8 Comments
Découvrez la meilleure pharmacie en ligne à prix réduit en un clic
April 1, 2024Options d’achat de danazol 50 mg en Belgique pharmacie
en ligne danazol 50 mg Espagne vente
danazol 50 mg disponible en ligne avec livraison rapide Trouvez facilement
danazol en ligne
indication du danazol 50 mg en vente au Maroc acheter danazol 50 mg
sans problème Belgique
danazol 50 mg en ligne sans ordonnance en France
danazol 50 mg : Points à considérer lors de l’achat en ligne
Achetez danazol à prix abordable en ligne
achat de danazol 50 mg en Espagne danazol 50 mg : Le point sur
les achats en ligne
danazol 50 mg : Guide d’achat en ligne Acheter danazol 50 mg
en toute simplicité sur internet
danazol 50 mg : guide d’achat en ligne pour les consommateurs avertis
danazol 50 mg sans ordonnance Suisse
danazol pour une solution sûre et efficace
Obtenez votre danazol sans délai danazol 50 mg sans ordonnance nécessaire
danazol 50 mg authentique et sûre en ligne
Options d’achat pratiques pour danazol France acheter danazol 50 mg Belgique légal
Prix abordable pour danazol en Belgique
Bespaar geld met de goedkoopste online internetapotheek - klik hier
April 1, 2024aankoop van lamisil in Gent Veilig terbinafine kopen zonder doktersrecept
terbinafine snel verzonden
Bestel terbinafine online en geniet van het gemak van thuisbezorging
waar terbinafine te kopen
gemakkelijk lamisil kopen in Europa
terbinafine kopen zonder recept: snel en gemakkelijk
terbinafine vrij verkrijgbaar in Amsterdam terbinafine kopen zonder recept: snel en gemakkelijk
lamisil bestellen zonder medisch advies
terbinafine bestellen via betrouwbare webshop terbinafine kopen voor natuurlijke verlichting
lamisil te koop online terbinafine: betrouwbare oplossing zonder recept verkrijgbaar.
cefadroxil disponible sans prescription en ligne
April 2, 2024First off I would like to say great blog! I had a quick question that I’d like to ask if you do not mind.
I was curious to find out how you center yourself and clear your thoughts before
writing. I have had difficulty clearing my mind in getting my ideas out there.
I truly do take pleasure in writing however it just seems like the first 10 to 15 minutes tend to be
lost just trying to figure out how to begin.
Any ideas or hints? Thank you!
kytril zonder recept: Eenvoudig en snel te bestellen
April 5, 2024Hey there! I’m at work surfing around your blog from my new iphone!
Just wanted to say I love reading your blog and look forward to all your posts!
Keep up the fantastic work!
achat de imiquimod en Belgique
April 8, 2024I am sure this paragraph has touched all the
internet visitors, its really really pleasant piece of
writing on building up new weblog.
Guida all'acquisto di tolterodine senza ricetta
April 12, 2024Hey There. I found your blog using msn. This is a really well written article.
I will make sure to bookmark it and return to read more of your useful information. Thanks for the post.
I will certainly comeback.
cetirizine Belgique sans ordonnance nécessaire
April 12, 2024Keep on writing, great job!
zoritoler imol
July 6, 2024F*ckin¦ remarkable issues here. I am very happy to peer your post. Thanks so much and i’m looking forward to contact you. Will you kindly drop me a e-mail?