Electrolysis
- Electrolysis is a process through which compounds are decomposed into their elements by use of electricity. Ions must be free to move i.e. when an ionic substance is dissolved in water or when melted through heat.
Electrolytic cell
- Converts electrical energy into chemical energy.
-
It consists of;
- Electrolyte is an ionic compound which conducts electric current in molten or aqueous solution, being decomposed in the process.
- Electrode is a rod or plate where electricity enters or leaves electrolyte during electrolysis. Reactions occur at electrodes.
- Discharge is the removal of electrons from negative ions to form atoms or the gain of electrons of positive ions to become atoms.
- Anode is positive electrode connected to positive terminal of d.c. source. Oxidation occurs here. Anode loses negative charge as electrons flow towards the battery, leaving anode positively charged. This causes anion to discharge its electrons here to replace lost electrons and also, negative charge are attracted to positive charge.
- Cathode is negative electrode connected to negative terminal of d.c. source. Reduction occurs here. Cathode gains negative charge as electrons flow from the battery towards the cathode, making cathode negatively charged. This causes cation to be attracted and gains electrons to be an atom.
- Anion is negative ion. It’s attracted to anode.
- Cation is positive ion. It’s attracted to cathode.

Electrolysis of molten lead bromide
- Lead bromide (PbBr2) is an ionic compound in solid form (white powder)
- When PbBr2 is heated, it melts and dissociates, leaving (lead ions) Pb2+ and (bromide) Br– ions free to move.
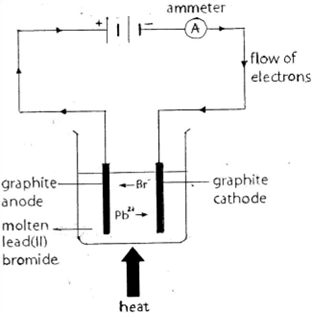
- At the cathode the lead ions (Pb2+) gains two electrons and are reduced to lead atoms which will form a silvery liquid metal at the bottom of the reaction vessel.

- At the anode bromide ions (Br–) lose electrons to form bromine atoms which combine to form molecules of bromine gas/vapour. Bubbles of brown gas (Br2) are seen at the anode.

2 Comments
Meilleurs sites pour commander aciphex en France
April 29, 2024Ridiculous quest there. What happened after?
Take care!
glyset: ghid de cumpărare online
May 6, 2024Heya i am for the first time here. I found this board and I find It truly
useful & it helped me out a lot. I hope to give something back and help others like you aided me.