Production of Ethanol
Fermentation of sugar or maize meal solution
- Fermentation is a chemical reaction in which sugars are broken down into smaller molecules such as ethanol by yeast in the absence of oxygen e.g. fermentation of glucose to form ethanol
- Yeast is added to a sugar solution and left at room temperature for a few days in the absence of air.
- Enzymes in yeast break down glucose to ethanol and carbon dioxide, giving out heat.
- Temperature must be kept between 25 and 35oC, above and below this temperature range, the enzymes become inactive.
- Sugar or sucrose is converted to glucose. Glucose is converted into ethanol and carbon dioxide
- Yeast is killed when the mixture contains more than 15% alcohol, therefore fermentation stops.
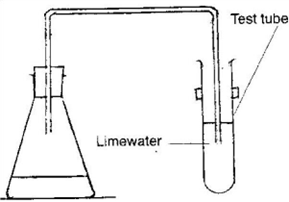

- Carbon dioxide is released during fermentation hence frothing is observed in the flask. Lime water changes from clear to milky.
- A dilute solution of ethanol is formed i.e. it is only about 15% concentration
Conditions for ethanol production
- pH of 6-8
- temperature of 25 – 37oC, higher temperature will denature enzymes
- anaerobic conditions
- enzymes in yeast
Production of concentrated ethanol
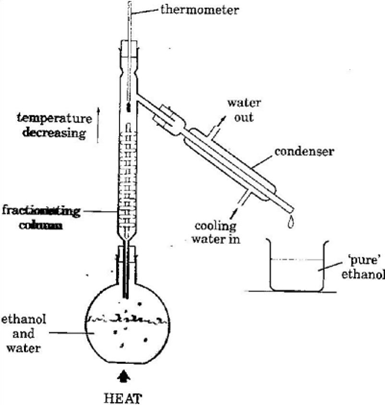
- Heat the mixture in the flask. At about 78oC the ethanol begins to boil. Some water evaporates too, so a mixture of ethanol and water vapour rises up the column.
- The vapour condenses on the glass beads in the column, making them hot.
- When the beads reach about 78oC, ethanol vapour no longer condenses on them. Only the water vapour does. So water drips back into the flask. The ethanol vapour goes into the condenser where it condenses into pure ethanol.
- Eventually, the thermometer reading rises above 78oC – a sign that all the ethanol has evaporated.
Uses of ethanol
-
- Beverages or alcoholic drinks e.g. beer or wine
- Solvent
- Medical purposes
- To produce cosmetics, detergents, plastics and lubricants
- Medical purposes
- Fuel