- Hydroxyl group makes carboxylic acids relatively unreactive towards nucleophilic acyl substitutions.
- The addition of a strong acid protonates the carbonyl oxygen making the carbonyl carbon more electrophilic.
- under the specific conditions carboxylic acids can be successfully converted into acid chlorides, acid anhydrides, esters, and amides through nucleophilic acyl substitution.
Conversion of Carboxylic acids to Acid Chlorides
- Carboxylic acids can be converted to acid chlorides by reaction with thionyl chloride (SOCl2).
- During the reaction with thionyl chloride, the hydroxyl group of the carboxylic acid is converted to an acyl chlorosulfite moiety which is a better leaving group.
- During the reaction a nucleophilic chloride anion is produced which reacts with the acyl chlorosulfite intermediate through nucleophilic acyl substitution to produce an acid halide.
General Reaction
Example
Mechanism
1) Nucleophilic attack on S=O bond
2) Removal of Cl leaving group
3) Nucleophilic attack on the carbonyl
4) Leaving group removal
5) Deprotonation
Acid Anhydride Formation
An acid anhydride is the product of condensation of two carboxylic acid molecules with the release of a water molecule eg acetic anhydride.
General Reaction
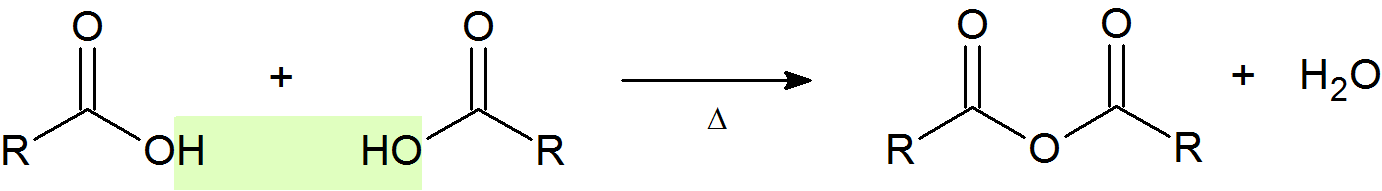
Example
Conversion of Carboxylic Acids into Esters by Alkylation
Carboxylic acids can be easily converted into their conjugate bases through deprotonation with a base, such as sodium hydroxide. The resulting carboxylate can be alkylated using by an SN2 reaction with either a methyl or primary halide. If a methyl ester is required, methyl iodide (CH3I) is a commonly used reagent.
Example
Conversion of Carboxylic Acids to Esters
General Reaction
Predicting the Products of a Fischer Esterification
Example
Mechanism
1) Protonation of the carbonyl
2) Nucleophilic attack on the carbonyl
3) Proton transfer
4) Removal of water as a leaving group
5) Deprotonation
Direct Conversion of Carboxylic Acids to Amides
General Reaction
Conversion of Carboxylic Acids to Amides using DCC
- During a DCC amide coupling, the OH of a carboxylic acid is replaced by an amine during nucleophilic acyl substitution.
- Using DCC as a coupling reagent, 1o and 2o amines can be used to create 2o and 3o amides respectfully.
Basic reaction
Predicting the products of DCC Coupling
Example
Mechanism
1) Deprotonation
2) Nucleophilic attack by the carboxylate
3) Nucleophilic attack by the amine
4) Proton transfer
5) Leaving group removal
Conversion of Carboxylic acids to 1o alcohols
Lithium aluminum hydride (LiAlH4)
- Hydride nucleophiles from lithium aluminum hydride (LiAlH4) can reduce carboxylic acids to 1o alcohols.
- NaBH4 is not a strong enough reducing agent to convert carboxylic acids or esters to alcohols.
General reaction
Predicting the product of a hydride reduction
Example
Mechanism
1) Deprotonation
2) Nucleopilic attack by a hydride anion
3) Leaving group removal
4) Nucleopilic attack by a hydride anion
5) Alkoxide protonation
1 Comment
Зарегистрироваться в binance
June 30, 2024I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.