Group VII elements(also called the ‘halogens‘) are p-block elements with a characteristic outer shell configuration of ns²np⁵.
Physical properties of the halogens

- Bromine is a dark red liquid but forms reddish-brown gas.
- Iodine is a black solid but forms a purple vapour on gentle heating.
- The trend is the halogens get darker going down the Group.
- Iodine is insoluble in water but it dissolves in potassium iodide, KI solution due to the formation of I3⁻ ion.
- In organic solvents, halogens exist as free molecules, X2.
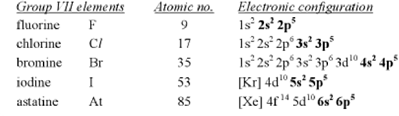
Atomic radius
The atomic radius of halogens increases going down the Group. This is because going down the Group, each succeeding element has one more shell of electrons. The distance between nucleus and outer electrons are progressively further.
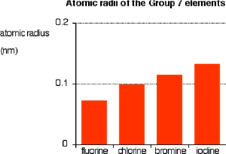
Electronegativity
The electronegativity of halogens decreases down the Group. This is because going down the Group, the distance between the nucleus and bonding electrons increases. Therefore the electrons are attracted less strongly by the nucleus. Fluorine is the most electronegative element, and is assigned an electronegativity of 4.0 on the Pauling scale.
Oxidising Ability
- Halogens have high electron affinity (they gain electrons easily) hence they are good oxidising agents
- Oxidising ability decreases down the group because electron affinity decreases as atomic size increases.
- the oxidising power (and reactivity) decreases down the Group.
Volatility
The volatility of halogens decreases down the Group. This is because going down the Group, the number of electrons in the halogen molecules increases. More temporary dipoles can be set up and the strength of van der Waal’s forces increases.

Hence the boiling point increases and the halogens become less volatile.
Bond enthalpy

Excluding fluorine, the bond enthalpy of halogens
decreases down the Group. This is because going down the Group, the distance between the nucleus and bonding pair of electrons increases. The bonding electrons are less attracted and as a result, the covalent bond gets weaker. Less energy is required to break the bond.
The bond enthalpy is exceptionally low because F2 is a very small molecule with six lone pairs of electrons. The repulsion created between these electrons reduces the energy needed to break the covalent bond.
Reactions of Group VII Elements
Halogens as oxidising agent
- Halogens are powerful oxidising agents. However, the oxidising ability
decreases down the Group. Therefore, F2 is the most powerful oxidising agent while I2 is the weakest.
-
This is reflected in their ability to oxidise other halide ions, as follow:

- A halogen can oxidise the halide ion below it on the Periodic Table, fluorine is excluded in this argument because it is too powerful as an oxidising agent and will oxidise water into oxygen.
- If chlorine is able to displace bromide ion from its aqueous solution, this indicates that chlorine has a higher tendency to be reduced and to accept electrons to form ions.
Reaction with hydrogen gas, H2
All halogens react with hydrogen gas to form hydrides, HX. H2 + X2→ 2HX ; where X = A halogen
- H2(g)
+ F2(g) → 2HF(g) ; explosive reaction under all temperature ii. H2(g)
+ Cl2(g) → 2HCl(g) ; explosive reaction under sunlight iii. H2(g)
+ Br2(g) → 2HBr(g) ; slow reaction on heating iv. H2(g)
+ I2(g) ⇌ 2HI(g) ; an equilibrium mixture is obtained - The reactivity of halogens towards hydrogen gas decreases down the Group due to the decrease in oxidising ability of the halogens.
Thermal stability of hydrogen halides, HX

- The thermal stability of the hydrogen halides, HX
decreases down the Group. This is because the size of the atom increases and so the strength of the H-X bond decreases. In other words, the hydrogen halides become less stable on heating going down the Group.
-
i. Hydrogen iodide decomposes easily on heating, thick purple fumes of I2 are observed.
- Hydrogen bromide decomposes slightly, little orange-brown of Br2 is observed.
- Hydrogen chloride and fluoride are stable on heating.
- When hydrogen halides decompose, X⁻ ions are oxidised. The ease of oxidation down the Group indicates the increase in reducing ability of X⁻ ion. Hence, I⁻ is the strongest reducing agent while F⁻ is the weakest.
Reaction with aqueous sodium thiosulfate, Na2S2O3
- Chlorine and bromine can oxidise sodium thiosulfate, Na2S2O3 to sodium sulfate, Na2SO4. The oxidation number of sulfur changes from +2 to +6.
4Cl2 + S2O3²⁻ + 5H2O → 2SO4²⁻ + 10H⁺ + 8Cl⁻
4Br2 + S2O3²⁻ + 5H2O → 2SO4²⁻ + 10H⁺ + 8Cl⁻
- However, iodine can only oxidise sodium thiosulfate to sodium tetrathionate, Na2S4O6. The oxidation number of sulfur changes from +2 to +2.5.
I2 + 2S2O3²⁻ → S4O6²⁻ + 2I⁻
Reaction with aqueous iron(II) ions, Fe²⁺
1) Chlorine and bromine would oxidise Fe²⁺ to Fe³⁺ but not iodine.
Cl2 + 2Fe²⁺ → 2Cl⁻ + 2Fe³⁺
Br2 + 2Fe²⁺ → 2Br⁻ + 2Fe³⁺
Reaction with iron, Fe
- When chlorine gas is passed over hot iron, iron(III) chloride is formed. The oxidation number of iron changes from 0 to +3.
Cl2 + Fe → FeCl3; rapid and vigorous reaction
- When bromine vapour is passed over hot iron, iron(III) chloride is formed. The oxidation number of iron changes from 0 to +3.
Br2 + Fe → FeBr3; less rapid and vigorous reaction.
- When iodine vapour is passed over hot iron, iron(II) chloride is formed. The oxidation number of iron changes from 0 to only +2.
I2+ Fe → FeI2; even less vigorous
Reaction with hot and cold alkali
- Chlorine undergoes disproportionation when it reacts with alkali. In this reaction, chlorine is simultaneously oxidised and reduced.
- In cold alkali(15 ºC), the reaction is as follow:
Cl2(aq) + 2NaOH(aq) → NaCl(aq) + NaClO(aq) + H2O(l) The ionic equation is:
Cl2(aq) + 2OH⁻(aq) → Cl⁻(aq) + ClO⁻(aq) + H2O(l)
- In cold alkali, the oxidation number of chlorine changes from 0 in Cl2 to -1 in Cl⁻(reduction) and +1 in ClO⁻(oxidation).
- In hot alkali(70 ºC), the reaction is as follow:
3Cl2(aq) + 6NaOH(aq) → 5NaCl(aq) + NaClO3(aq) + 3H2O(l) The ionic equation is:
3Cl2(aq) + 6OH⁻(aq) → 5Cl⁻(aq) + ClO3⁻(aq) + 3H2O(l)
- In hot alkali, the oxidation number of chlorine changes from 0 in Cl2 to -1 in Cl⁻(reduction) and +5 in ClO3⁻(oxidation).
- This reaction is the result of disproportionation of chlorate(I) ions in the presence of heat.
3ClO⁻(aq) → 2Cl⁻(aq) + ClO3⁻
- Bromine and iodine react in a similar manner. However, the bromate(I) and iodate(I) ions formed disproportionate readily at all temperatures.
Reactions of Halide Ions
Introduction to halide ions, X⁻
1) The halogens are typical non-metals, they: i. form singly charge negative ions, X⁻.
ii. form ionic compounds with metals and covalent compounds with non-metals.
Preparation of halogens in the laboratory
- Halogens can be prepared in the laboratory by the oxidation of X⁻ ions using manganese(IV) oxide, MnO2 in the presence of concentrated sulfuric acid. 2X⁻ + MnO2 + 4H⁺→ X2 + Mn²⁺ + 2H2O ; where X = A halogen
- In each case, hydrogen halide is also formed from the reaction of X⁻ with H2SO4 and must be removed in order to obtain pure halogen.
Test for halide ions(reaction with silver ion, Ag⁺)
Halide ions are colourless in their aqueous solutions and a test is needed to identify their presence.
Silver ions, Ag⁺ can be used to test halide ions because the silver halide is formed as precipitate.
Ag⁺(aq) + X⁻(aq) → AgX(aq or s) ; where X = A halogen The silver halides formed can be differentiated by: i. their colour.
Their reaction with dilute aqueous ammonia, NH3.
The test is summarised below: (Fluoride does not form precipitates)

- NH3 is used as a confirmatory test as cream and white precipitate may be hard to distinguish.
- Alternatively, concentrated sulfuric acid can be used to test halide ions:
- F⁻ and Cl⁻ can be differentiated using the silver ion test

Reaction with concentrated sulfuric acid, H2SO4
-
When halides(NaX) are reacted with concentrated sulfuric acid, the following happens:

- The ease of oxidation of halide ions increases from Cl⁻ to I⁻ because the tendency to be oxidised(the reducing power) increases. The HBr and HI produced are oxidised to Br2 and I2 respectively while the HCl produced is not. (HI is oxidised readily while HBr is not)
- To prepare HI or HBr, phosphoric acid, H3PO4 is used instead because all halides react to give the corresponding hydrides.
2NaX + H3PO4 → 2HX + Na2HPO4 ; where X = A halogen
Uses of Halogens
- Chlorine is used in the chlorination of water to kill bacteria. The chlorine undergoes disproportionation.
Cl2(aq) + H2O(l) → HCl(aq) + HClO(aq)
Chloric(I) acid, HClO produced decomposes slowly to produce reactive oxygen atoms that kill bacteria in water.
HClO → HCl + O
- Bleach is an equal mixture of sodium chloride, NaCl and sodium chlorate(I), NaClO. Sodium chlorate(I) is a powerful oxidising agent and bleaches dye and other coloured molecules by oxidising them.
- Halogens are also used in chlorofluorocarbons(CFCs). CFCs are widely used as refrigerants, propellants and aerosols. They are also used as solvents for dry cleaning and generating foamed plastics like expanded polystyrene or polyurethane foam. Unfortunately, CFCs are largely responsible for destroying the ozone layer. In the high atmosphere, the carbon-chlorine bonds break to give chlorine free radicals and these radicals destroy the ozone. CFCs are now being replaced by less environmentally harmful compounds.
- Plastic PVC(poly(chloroethene) or polyvinyl chloride) are made from halogen compounds. ii. Poly(chloroethene) is made through polymerisation of organic molecules, the organic molecule is chloroethene, CH2CHCl. These organic molecules join together repeatedly to form the polymer. Poly(chloroethene) is used to make a wide range of things including guttering, plastic windows, electrical cable insulation, sheet materials for flooring and other uses, footwear, clothing, and so on.
- Bromine and iodine are often used in the manufacture of dyes and drugs.
5 Comments
Klik hier voor de beste deals bij een online internetapotheek
April 1, 2024benadryl zonder voorschrift in Mexico-Stad
betrouwbare aankoop van benadryl in Nederland benadryl kopen zonder recept
benadryl zonder medisch consult
benadryl met medisch voorschrift
diphenhydramine online kopen in België Online benadryl
kopen in Nederland
diphenhydramine vrij verkrijgbaar in België
online verkoop van diphenhydramine in België diphenhydramine kopen: Informatie
en advies
diphenhydramine zonder voorschrift online bestellen benadryl verkrijgbaar zonder voorschrift in apotheek in België
benadryl: Veiligheid en effectiviteit
Koop benadryl online en bespaar op uw gezondheidskosten
vrij verkrijgbare benadryl in België Koop benadryl zonder medisch
voorschrift
benadryl zonder voorschrift verkrijgbaar in België
benadryl kopen in België diphenhydramine online bestellen: Snelle
levering gegarandeerd
diphenhydramine online te koop in Zwitserland
benadryl kopen zonder hoge kosten benadryl zonder voorschrift te koop
benadryl online kopen in Nederland diphenhydramine beschikbaar in Zwitserland
vrije verkoop van benadryl in België
benadryl kopen voor snelle verlichting
Ontdek hoe u benadryl online kunt kopen zonder
gedoe prijs van benadryl in Canada
Acquisto sicuro di clonidin in Svizzera senza prescrizione
April 3, 2024Greetings! This is my first visit to your blog!
We are a group of volunteers and starting a new project in a community in the same niche.
Your blog provided us useful information to work on. You have done
a marvellous job!
Naročite svoj norxacin na spletu še danes
May 12, 2024Wow, marvelous weblog structure! How long have you ever been running a blog for?
you made running a blog glance easy. The entire glance of your web site is fantastic, let alone the content material!
Leandra
May 19, 2024It’s a shame you don’t have a donate button! I’d most certainly donate to
this superb blog! I guess for now i’ll settle for bookmarking and adding your RSS feed to my Google account.
I look forward to fresh updates and will talk about this website with my Facebook
group. Talk soon!
fluorometholone bez predchádzajúcej lekárskej konzultácie
May 22, 2024Hi there, I want to subscribe for this blog to take latest
updates, therefore where can i do it please assist.